Abstract
Background: The role of remdesivir in the treatment of hospitalized patients with COVID-19 remains ill-defined. We conducted a cost-effectiveness analysis alongside the Canadian Treatments for COVID-19 (CATCO) open-label, randomized clinical trial evaluating remdesivir.
Methods: Patients with COVID-19 in Canadian hospitals from Aug. 14, 2020, to Apr. 1, 2021, were randomly assigned to receive remdesivir plus usual care versus usual care alone. Taking a public health care payer’s perspective, we collected in-hospital outcomes and health care resource utilization alongside estimated unit costs in 2020 Canadian dollars over a time horizon from randomization to hospital discharge or death. Data from 1281 adults admitted to 52 hospitals in 6 Canadian provinces were analyzed.
Results: The total mean cost per patient was $37 918 (standard deviation [SD] $42 413; 95% confidence interval [CI] $34 617 to $41 220) for patients randomly assigned to the remdesivir group and $38 026 (SD $46 021; 95% CI $34 480 to $41 573) for patients receiving usual care (incremental cost −$108 [95% CI −$4953 to $4737], p > 0.9). The difference in proportions of in-hospital deaths between remdesivir and usual care groups was −3.9% (18.7% v. 22.6%, 95% CI −8.3% to 1.0%, p = 0.09). The difference in proportions of incident invasive mechanical ventilation events between groups was −7.0% (8.0% v. 15.0%, 95% CI −10.6% to −3.4%, p = 0.006), whereas the difference in proportions of total mechanical ventilation events between groups was −5.7% (16.4% v. 22.1%, 95% CI −10.0% to −1.4%, p = 0.01). Remdesivir was the dominant intervention (but only marginally less costly, with mildly lower mortality) with an incalculable incremental cost effectiveness ratio; we report results of incremental costs and incremental effects separately. For willingness-to-pay thresholds of $0, $20 000, $50 000 and $100 000 per death averted, a strategy using remdesivir was cost-effective in 60%, 67%, 74% and 79% of simulations, respectively. The remdesivir costs were the fifth highest cost driver, offset by shorter lengths of stay and less mechanical ventilation.
Interpretation: From a health care payer perspective, treating patients hospitalized with COVID-19 with remdesivir and usual care appears to be preferrable to treating with usual care alone, albeit with marginal incremental cost and small clinical effects. The added cost of remdesivir was offset by shorter lengths of stay in the intensive care unit and less need for ventilation.
Study registration: ClinicalTrials. gov, no. NCT04330690
The role of remdesivir in treating hospitalized patients with COVID-19 remains uncertain.1–3 Remdesivir, a repurposed antiviral medication, has received regulatory approval from Health Canada for treatment of patients with COVID-19 pneumonia requiring supplemental oxygen. This was based on clinical trial data documenting faster time to recovery.4 Remdesivir’s impact on other clinical outcomes, including deaths and post-hospitalization outcomes, has yet to be determined.5 Recommendations have varied for or against the use of remdesivir in patients with COVID-19 by different governing bodies.1–3
The World Health Organization (WHO) Solidarity trial5 is a global, pragmatic clinical trial examining the effects of various therapeutics in COVID-19, with final remdesivir results pending publication.5 Canadian Treatments for COVID-19 (CATCO) is the Canadian arm of the Solidarity trial, which showed that use of remdesivir led to a modest but significant reduction in the need for mechanical ventilation, but not a significant difference in hospital mortality.6 There are substantial drug acquisition costs for remdesivir; therefore, a health economic evaluation is an important component in health care system decision-making for its use. Other jurisdictions have showed cost-effectiveness or dominance of remdesivir compared with supportive care,7 although most studies were model-based.8,9
We conducted a cost-effectiveness analysis (E-CATCO) alongside the CATCO trial assessing remdesivir plus usual care versus usual care alone by measuring health care resource utilization and costs and clinical outcomes in the 2 treatment arms for hospitalized adults with COVID-19.
Methods
The primary objective of E-CATCO was to estimate the incremental costs per survivor associated with the use of remdesivir plus usual care (remdesivir group) versus usual care alone (usual care group) in patients hospitalized with COVID-19. Our secondary objective was to assess the cost-effectiveness of preventing 1 episode of invasive mechanical ventilation.6 We performed the economic evaluation from the public health care payer’s perspective, over the time horizon from randomization to discharge or in-hospital death (Table 1).10
Summary of health economic evaluation framework (E-CATCO)
We developed the economic evaluation according to cost-effectiveness analysis recommendations,11 including from the Canadian Agency for Drugs and Technologies in Health (CADTH),12 and the Consolidated Health Economic Evaluation Reporting Standards (CHEERS)13 checklist (Appendix 1, Supplemental Table 1, available at www.cmajopen.ca/content/10/3/E807/suppl/DC1). The CATCO trial was conducted in accordance with Good Clinical Practice guidelines of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use.14 A priori informed or deferred consent for participation in the CATCO trial was obtained from each trial participant or their substitute decision-maker.6
Patients
CATCO was a pragmatic, adaptive, multicentre randomized controlled trial, in which multiple agents were compared against the available standard of care in an open-label fashion. Detailed eligibility criteria are described elsewhere.6
Eligible patients were randomized in a 1:1 ratio to remdesivir plus usual care (remdesivir group) versus usual care alone (usual care group) during periods when remdesivir was available. Patients randomly assigned to remdesivir received 200 mg intravenously initially, followed by 100 mg intravenously for up to 9 additional days (or until discontinued by the treatment team at the physician’s discretion or hospital discharge, whichever came first) plus usual care. Patients randomly assigned to the control arm received usual care without remdesivir. Usual care was dynamic but left to local standards and the treating clinician, including co-interventions, such as dexamethasone, therapeutic anticoagulation and tocilizumab. Patients were discharged when they were deemed clinically eligible by the treating team, and the study intervention was stopped if this was before completion of a full treatment course.6 Patient follow-up was until hospital discharge or death.
From Aug. 14, 2020, to Apr. 1, 2021, 1282 adult, hospitalized, laboratory-confirmed COVID-19 patients (full CONSORT [Consolidated Standards of Reporting Trials] diagram presented elsewhere) were randomized in CATCO (remdesivir domain).6 Patients were enrolled in 1 of 52 hospitals from 6 Canadian provinces (British Columbia, Alberta, Manitoba, Ontario, Quebec, Newfoundland and Labrador). We determined unit costs after the last patient was recruited in CATCO and before conducting the primary CATCO outcome analyses. One patient was excluded from analyses owing to incomplete data collection. In the final analysis, 1281 patients were included: 634 in the remdesivir group and 647 in the usual care group.6
Clinical outcomes
We collected the clinical effects, frequencies or proportions, and per-patient event rates for all randomized patients as part of the CATCO trial, and analyzed with the intention-to-treat principle. The primary clinical outcome of this economic evaluation was the incremental difference in hospital mortality. The secondary clinical outcome was episodes of invasive mechanical ventilation. Given the in-hospital time horizon and emphasis on mortality, health-related quality of life was not measured, and we did not estimate quality-adjusted life-years or extrapolate lifetime outcomes.
Unit costs and health resource use
Based on a predefined list of items (based on CATCO case-report forms), the E-CATCO steering committee reviewed the relative importance of cost variables before analysis to guide the number of variables included. Similar methodology has been described elsewhere.15
A line-item list of unit costs and health care resource use was devised, using categories including the following: medications, personnel, diagnostic radiology and laboratory testing, operations and procedures, and per-day hospital (e.g., hoteling) costs not otherwise encompassed, in accordance with recommendations on measuring resource use.10,16–18 Total costing (resource use multiplied by unit cost) methodology is similarly described elsewhere.15 Duplicate disaggregate unit costs reported at a site level were removed, to avoid double-counting.15
We preferentially recorded unit costs published by public health care payers (e.g., provincial schedule of benefits, formularies from jurisdictions) as an estimation of unit costs.12 A jurisdiction was defined as a region (e.g., province) that is responsible for the costing and delivery of health care.12 For unit costs not available through the public sources, we extracted unit costs from the main study site (Sunnybrook Hospital). We obtained costing data with assistance from hospital unit managers, accounting, human resources, pharmacy, radiology or laboratory departments, where possible. If a specific line-item unit cost was not attainable for a specific jurisdiction, we used a mean unit cost approach for the jurisdictions that reported unit costs.15,19,20
Costing, primary cost-effectiveness analysis, and subgroup and sensitivity analyses
We used descriptive epidemiologic analyses, including means (with standard deviations [SDs]), counts and proportions to describe baseline characteristics, effect and cost estimates. We adjusted all costs to 2020 Canadian dollars.21 Continuous data were presented as means and SDs or medians and interquartile ranges (IQRs), which were compared (where appropriate) using a t-test or Wilcoxon rank-sum test, with 95% confidence intervals (CIs) for differences in means reported, where applicable. Categorical variables and proportions were compared using the Pearson χ2 or Fisher exact tests as appropriate.
For our base-case primary analysis, individual resource utilization was multiplied by jurisdiction unit costs to calculate individual patient total costs. We estimated appropriate “standard dose” for nontitrated medications (e.g., antibiotics) and a clinically appropriate “medium dose” for various titratable medications (e.g., vasoactive medications, sedatives and analgesics, neuromuscular blockers). Appendix 2, Supplemental Table 2, available at www.cmajopen.ca/content/10/3/E807/suppl/DC1, outlines assumptions for estimating other resource utilization.
Incremental cost-effectiveness ratios for primary outcome of mortality and secondary outcome of invasive mechanical ventilation averted (mean cost and effects, per patient) in E-CATCO
We calculated total costs for remdesivir and usual care groups by summing each of the individual patient costs, and we then divided by the number of patients in each group to calculate the mean cost per patient for each group. Incremental costs were defined as the difference in mean per-patient costs between groups and incremental effects as the difference in proportions of clinical outcomes between groups (given differing sample sizes between groups).15
The incremental cost-effectiveness ratio (ICER) measured the ratio of incremental costs of the remdesivir group versus the usual care group per incremental clinical outcome (e.g., death, invasive mechanical ventilation event averted).10,13 If ICERs were negative, incremental costs and incremental effects were reported separately. Similar methodology has been previously described.15,19,20
We conducted prespecified subgroup analyses (as outlined in CACTO) according to age (< 55 yr, ≥ 55 yr), sex (male, female) and illness severity at randomization based on the WHO Ordinal Scale.5,6 Multivariable linear regression and logistic regression modelling were used to determine the significance of any interactions between subgroups for cost and mortality, respectively.
To assess the uncertainty associated with cost and effects estimation, we used nonparametric bootstrapping with replacement techniques. We generated 1000 simulated costs and effects for individual patients, for remdesivir plus usual care and usual care alone groups, for all outcomes. Each of the 1000 bootstraps were to the size of the original sample within each treatment arm (remdesivir and control). These were plotted on cost-effectiveness planes. Cost-effectiveness acceptability curves were used to present the probability of remdesivir being cost-effective over a wide range of willingness-to-pay thresholds. A tornado diagram was constructed to describe the major cost drivers.10
We performed multiple sensitivity analyses with variations of estimates of pairs of potentially influential variables (e.g., ranges of remdesivir cost, hoteling costs, and intensive care unit [ICU] and ward nursing ratios) across plausible ranges to determine whether different estimates changed the overall results. All analyses were performed using Excel version 14.0.6 (Microsoft Corp) and SAS version 9.4.
Ethics approval
This study was approved by local research ethics boards and coordinated by Sunnybrook Research Institute at the University of Toronto.
Results
The characteristics of the patients included in the E-CATCO study are as published in the main CATCO trial report.6 The full cost-effectiveness analysis data set (including cost-effectiveness planes, cost-effectiveness acceptability curves and nonparametric bootstrap sampling) are available on request from the corresponding author.
Clinical outcomes and incremental effects
The difference in proportions of in-hospital deaths between remdesivir and usual care groups was −3.9% (18.7% remdesivir v. 22.6% usual care, 95% CI −8.3% to 1.0%, p = 0.09). The difference in proportions of incident invasive mechanical ventilation events between groups was −7.0% (8.0% remdesivir v. 15.0% usual care, 95% CI −10.6% to −3.4%, p < 0.006), whereas the difference in proportions of total invasive mechanical ventilation events between groups was −5.7% (16.4% remdesivir v. 22.1% usual care, 95% CI −10.0% to −1.4%, p = 0.01). The difference in ICU length of stay was as follows: 2340 (remdesivir) versus 3045 (usual care) total days, absolute difference −705 days, mean 3.7 (SD 6.8) days (remdesivir) versus 4.7 (SD 8.1) days (usual care), mean difference −1.0 days per patient (95% CI −0.2 d to −1.8 d, p = 0.02) (Table 2).6
Health care resource use and costs
Resource utilization and mean unit cost are outlined in Table 3. The mean cost per patient was $37 918 (SD $42 413, 95% CI $34 617 to $41 220) for the remdesivir group, and the mean cost per patient was $38 026 (SD 46 021, 95% CI $34 480 to $41 573) for the usual care group. The incremental cost per patient was −$108 (SD $62 584, 95% CI −$4953 to $4737, p > 0.9) (Table 3).
Study resource utilization and mean unit costs
Cost-effectiveness, subgroup and sensitivity analyses
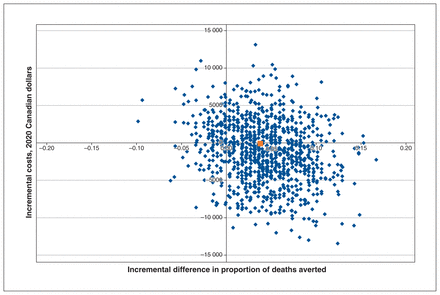
In the base-case primary analysis of death averted with remdesivir versus usual care (Table 2), the ICER was incalculable owing to dominance of remdesivir (marginally less costly, marginally more effective) over usual care alone on the cost-effectiveness plane (Figure 1; Table 2). The ICERs and cost-effectiveness plots for invasive mechanical ventilation (secondary objective) are presented in Table 3 and Appendix 3, Supplemental Figure 1, available at www.cmajopen.ca/content/10/3/E807/suppl/DC1. The ICER for invasive mechanical ventilation was also incalculable owing to dominance of remdesivir (marginally less costly and more effective).
Incremental cost-effectiveness plane for deaths averted (remdesivir v. placebo — with usual care): point estimate (red) and nonparametric bootstrapping simulations (blue).
Reported separately, incremental costs were −$108 (95% CI −$4953 to $4737, p > 0.9), in favour of remdesivir. Incremental effects for mortality were −3.9% (18.7% v. 22.6%, 95% CI 8.3% to 1.0%, p = 0.09), in favour of remdesivir. Incremental effects for invasive mechanical ventilation were −7.0% (8.0% v. 15.0%, 95% CI −10.6% to −3.4%, p = 0.006), in favour of remdesivir. The difference in proportions of total mechanical ventilation events between groups was −5.7% (16.4% v. 22.1%, 95% CI −10.0% to −1.4%, p = 0.01).
The cost-effectiveness acceptability curves are presented in Figure 2 for mortality. Across a willingness-to-pay threshold of $0, $20 000, $50 000 and $100 000 per death averted, a strategy using remdesivir was economically attractive in 60%, 67%, 74% and 79% of simulations, respectively (Figure 2).
Cost-effectiveness acceptability curve for deaths averted (remdesivir v. placebo — with usual care) for varying willingness-to-pay thresholds.
Cost-effectiveness acceptability curves for prevention of invasive mechanical ventilation are shown in Appendix 4, Supplemental Figure 2, available at www.cmajopen.ca/content/10/3/E807/suppl/DC1. Across a willingness-to-pay threshold of $0, $20 000, $50 000 and $100 000 per invasive mechanical ventilation averted, a strategy using remdesivir was economically attractive in 58%, 66%, 75% and 82% of simulations, respectively.
Our prespecified subgroup analyses (age, sex and illness severity on admission by WHO Ordinal Scale) showed no significant subgroup interactions for cost, except for a significant improvement in survival and lower costs among patients older than 55 years treated with remdesivir (Appendix 5, Supplemental Table 3, available at www.cmajopen.ca/content/10/3/E807/suppl/DC1).
In sensitivity analyses, cost-neutrality (based on ~$0 willingness-to-pay threshold) for remdesivir is achieved at the base-case Can$2925 per patient course. However, if the price of remdesivir were increased to $3791 (increase of $866/patient), $4928 (increase of $2003/patient) and $6823 (increase of $3898/patient) per course, the willingness-to-pay threshold would increase the ICERs for those scenarios to $20 000, $50 000 and $100 000 per death averted, respectively.
Our base-case analysis kept patient to nursing ratios at 1:1 in ICU and 4:1 on the ward (incremental costs: −$108 per patient). However, to address the finding that the incremental costs were heavily influenced by expense of care in the ICU, we explored less expensive ICU staffing models. If patient to nurse ratios changed to ICU 1.5:1 and ward 5:1, incremental costs increased $196 (SD $59 327) (difference of +$304) as compared with the base case, and had a calculable ICER of $5178 per death averted. If ratios changed to ICU 1.5:1 and ward 6:1, incremental costs increased $161 (SD $59 363) (difference of +$269) with an ICER of $4246 per death averted.
In our base-case analysis, mean ICU hoteling costs were $3495 among all jurisdictions (incremental costs: −$108). If ICU hoteling were reduced to $2000 (replicating less expensive health care systems), incremental costs would increase by $722 (increase of +$830 per patient) (ICER: $19 061 per death averted). If ICU hoteling were increased to $5000 (replicating more expensive health care systems), incremental costs would decrease by −$2257 (decrease −$2365 per patient), where remdesivir was dominant.
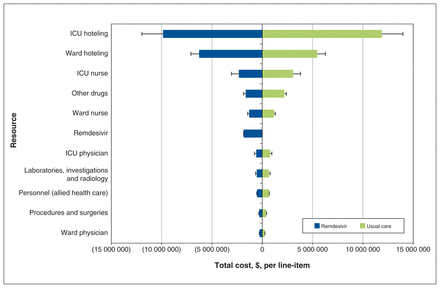
An aggregated tornado diagram (Figure 3) and full tornado diagram (Appendix 6, Supplemental Figure 3, available at www.cmajopen.ca/content/10/3/E807/suppl/DC1) show the major cost drivers in E-CATCO. The top 5 major cost drivers were ICU hoteling, ward hoteling, ICU nursing, other drugs (all lower in remdesivir group) and remdesivir drug cost (higher in remdesivir group). Cost distributions per group are shown by box plots (Appendix 7, Supplemental Figure 4, available at www.cmajopen.ca/content/10/3/E807/suppl/DC1).
Tornado diagram of major cost drivers in E-CATCO (summarized by major costing categories). CATCO = Canadian Treatments for COVID-19, E-CATCO = economic evaluation alongside CATCO, ICU = intensive care unit.
Interpretation
In this health economic evaluation performed alongside the CATCO clinical trial, we found that remdesivir plus supportive care is likely the preferred treatment strategy (similar-to-lower costs with similar-to-increased survival and less need for mechanical ventilation) compared with usual care alone, for hospitalized adults with COVID-19. Lower costs associated with a treatment strategy using remdesivir were predominantly from reductions in ICU hoteling, ward hoteling, ICU nursing, ward nursing and use of other drugs, despite the drug acquisition cost for remdesivir.
Our findings from E-CATCO provide economic context to the clinical effects of remdesivir.6 Although the reduction in mortality with remdesivir treatment was nonsignificant, a reduced need for new mechanical ventilation and ICU resources led to lower resource utilization, which offset remdesivir drug costs. Our economic findings augment the findings regarding the clinical effects of remdesivir for adult, hospitalized patients with COVID-19.1–3,6 These findings are consistent with cost-effectiveness analyses of remdesivir in the literature from other jurisdictions and studies using model-based designs.7–9
Despite similar mean and median hospital length of stay in the 2 arms of CATCO,6 there were meaningful reductions in the remdesivir group for both new need for mechanical ventilation and total days in ICU. The additional time in ICU for patients receiving usual care only was the largest incremental cost driver. This analysis exemplifies how numerically but nonsignificant clinical differences in length of stay may still have an important impact on incremental cost estimation in a health economic evaluation.15,19
This economic analysis also highlights the value of considering clinical effectiveness alongside costs and resource use. Every dollar spent for a nonbeneficial or cost-ineffective intervention is an opportunity cost lost for other interventions in a health system with finite resources, with the potential for indirect harms to other patients.22
Finally, it is important to emphasize that we focused on a base-case patient hospitalized with COVID-19, finding treatment with remdesivir likely to be economically attractive (albeit marginally). There may be signals of increased clinical benefit and lower costs in patients older than 55 years.
Upstream strategies to prevent infection and hospitalization (e.g., infection prevention through public health measures, including vaccination) are generally the most effective strategies in improving health outcomes and lowering costs that health systems have at their disposal.23–27 Accordingly, health policy-makers and clinicians also need to consider expenditures on upstream and downstream resource use and medications from this broader population-based budgetary perspective.
There are several strengths of this study. It was conducted in accordance with cost-effectiveness analysis guidelines, and CADTH and CHEERS recommendations,11–13,18 using similar methodology to other cost-effectiveness analyses conducted alongside trials in the Canadian context.15,19 Clinical effects and costs are based on patient-level data from a randomized trial rather than model-based, hypothetical cohorts. We also incorporated inputs from multiple sources, increasing the internal validity for both costs and effects. Capturing jurisdictional costs and effects with their own distributions and variance allowed for a more precise estimate of between-group differences, which enhances the generalizability of these findings.
Limitations
This analysis also has limitations. The short time horizon (randomization to in-hospital discharge or death) may miss additional costs associated with downstream health consequences secondary to COVID-19. Unfortunately, we could not capture effects or costs past hospital discharge in multiple jurisdictions accurately. This is potentially important owing to a proportion of patients having long-lasting COVID-19 symptoms.28,29 This health economic evaluation derived data from a randomized trial and may not represent the same treatment effects and costs as in routine clinical practice,15 although CATCO is pragmatically designed. Finally, future research gaps to be addressed include differences in costs and effects of age older than 55 years, 5 days versus 10 days of remdesivir therapy,30 timing of remdesivir initiation after symptom onset, influence of vaccination status and new variants on remdesivir effectiveness, and unit cost estimation at different time points (e.g., the drug acquisition cost of remdesivir could be less over time). External validity is limited to the Canadian health care perspective but is likely comparable to many other third-party payer jurisdictions.
Conclusion
From a health care payer perspective, treating hospitalized patients with COVID-19 with remdesivir and usual care is likely preferrable to treating with usual care alone, albeit with marginal incremental cost and small clinical effects. Cost-effectiveness analyses should be included as a component of the evaluation of medications for patients with COVID-19.
Acknowledgements
E-CATCO was designed by the CATCO steering committee and the Canadian Critical Care Trials Group. Special thanks to Drs. David Williamson and Claudio Martin for their reviews of this manuscript. The authors are grateful for the commitment of all their colleagues in participating centres and staff at the Sunnybrook Research Institute for their expertise.
Footnotes
Competing interests: Robert Fowler is the H. Barrie Fairley Professor of Critical Care Medicine at the University Health Network and the University of Toronto Interdepartmental Division of Critical Care Medicine. Robert Fowler declares a Canadian Institutes of Health Research (CIHR) operating grant. John Conly declares grants from the CIHR, Pfizer and the World Health Organization (WHO). He declares a peer-reviewed research grant on acute and primary care preparedness for COVID-19 in Alberta, Canada; he was a primary local investigator for the STRIVE Staphylococcus aureus vaccine randomized controlled trial in vertebral spinal surgery with instrumentation for which all funding was provided only to the University of Calgary; he was a co-investigator on a WHO-funded study using integrated human factors and ethnography approaches to identify and scale innovative infection prevention and control (IPC) guidance implementation supports in primary care with a focus on low-resource settings and using drone aerial systems to deliver medical supplies and personal protective equipment to remote First Nations communities during the COVID-19 pandemic. John Conly also reports receiving accommodations and airfare from the Centers for Disease Control and Prevention to attend a meeting in 2019. He is a member and chair of the WHO Infection Prevention and Control Research and Development Expert Group for COVID-19 and a member of the WHO Health Emergencies Programme Ad-hoc COVID-19 IPC Guidance Development Group, both of which provide multidisciplinary advice to the WHO, for which no funding is received and from which no funding recommendations are made for any WHO contracts or grants. He is also a member of the Cochrane Acute Respiratory Infections Group. Darrell Tan is supported by a Tier 2 Canada Research Chair in HIV Prevention and STI Research. Ryan Zarychanski reports grants from the CIHR, the Peter Munk Cardiac Centre, the Thistledown Foundation and the National Institutes of Health. He is a WHO thrombostasis technical advisory member. Ryan Zarychanski is the recipient of the Lyonel G. Israels Research Chair in Hematology at the University of Manitoba. Todd Lee reports a CATCO operating grant from the CIHR as a co–principal investigator and a co-investigator. He reports various operating grants from the CIHR, a technical development grant from the Centre for Aging + Brain Health Innovation and research salary support from the Fonds de recherche du Québec — Santé. He is the co-owner of a company that is bringing Med-Safer to market. Srinivas Murthy is the Innovative Medicines Canada and Health Research Foundation Chair in Pandemic Preparedness Research. Srinivas Murthy reports a grants from the CIHR and Health Research Foundation and Innovative Medicines Canada.
This article has been peer reviewed.
Contributors: Vincent Lau, Robert Fowler, Ruxandra Pinto, François Carrier, Matthew Cheng, John Conly, Cecilia Costiniuk, Erick Duan, Madeleine Durand, Patricia Fontela, George Farjou, Mike Fralick, Anna Geagea, Jennifer Grant, Kosar Khwaja, Nelson Lee, Todd Lee, Rachel Lim, Conar O’Neil, Jesse Papenburg, Makeda Semret, Michael Silverman, Wendy Sligl, Ranjani Somayaji, Darrell Tan, Jennifer Tsang, Jason Weatherald, Cedric Yansouni, Ryan Zarychanski and Srinivas Murthy all have made substantial contributions to the conception and design, acquisition of data, analysis and interpretation of data, and drafted the submitted article and revised it critically for important intellectual content. Vincent Lau, Robert Fowler, Srinivas Murthy and Ruxandra Pinto contributed to the conception and the background. Vincent Lau, Robert Fowler, Ruxandra Pinto, François Carrier, Matthew Cheng, John Conly, Cecilia Costiniuk, Erick Duan, Madeleine Durand, Patricia Fontela, George Farjou, Mike Fralick, Anna Geagea, Jennifer Grant, Kosar Khwaja, Nelson Lee, Todd Lee, Rachel Lim, Conar O’Neil, Jesse Papenburg, Makeda Semret, Michael Silverman, Wendy Sligl, Ranjani Somayaji, Darrell Tan, Jennifer Tsang, Jason Weatherald, Cedric Yansouni, Ryan Zarychanski and Srinivas Murthy contributed to the data collection. Vincent Lau, Robert Fowler, Ruxandra Pinto and Srinivas Murthy contributed to the data analysis. Vincent Lau, Robert Fowler, Ruxandra Pinto, François Carrier, Matthew Cheng, John Conly, Cecilia Costiniuk, Erick Duan, Madeleine Durand, Patricia Fontela, George Farjou, Mike Fralick, Anna Geagea, Jennifer Grant, Kosar Khwaja, Nelson Lee, Todd Lee, Rachel Lim, Conar O’Neil, Jesse Papenburg, Makeda Semret, Michael Silverman, Wendy Sligl, Ranjani Somayaji, Darrell Tan, Jennifer Tsang, Jason Weatherald, Cedric Yansouni, Ryan Zarychanski and Srinivas Murthy contributed to drafting the manuscript. Vincent Lau, Robert Fowler, Ruxandra Pinto, François Carrier, Matthew Cheng, John Conly, Cecilia Costiniuk, Erick Duan, Madeleine Durand, Patricia Fontela, George Farjou, Mike Fralick, Anna Geagea, Jennifer Grant, Kosar Khwaja, Nelson Lee, Todd Lee, Rachel Lim, Conar O’Neil, Jesse Papenburg, Makeda Semret, Michael Silverman, Wendy Sligl, Ranjani Somayaji, Darrell Tan, Jennifer Tsang, Jason Weatherald, Cedric Yansouni, Ryan Zarychanski and Srinivas Murthy contributed to revising the manuscript. Alain Tremblay, Sergio Borgia, Peter Daley and Yoav Keenan all contributed to the design of the work and drafting of work for important intellectual content. All authors provided final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This economic evaluation (E-CATCO) and CATCO were funded by the Canadian Institutes of Health Research, the Vancouver Coastal Health Research Institute, the Northern Alberta Clinical Trials and Research Centre, the Covenant Health Research Centre, the St. Joseph’s Healthcare Foundation and the London Health Sciences Foundation. No funding for either the trial itself or this economic evaluation was received from the manufacturers of remdesivir (Gilead) or other treatments, or from sources not listed above. None of the funders played a role in the conception, design, conduct, oversight, analysis or interpretation of the work, in the decision to submit this manuscript for publication, or in the preparation, review or approval of the manuscript.
Oversight: Study methods, operations and manuscript generation were coordinated by the E-CATCO steering committee (Vincent Lau, Robert Fowler, Srinivas Murthy, Alain Tremblay and Ruxandra Pinto).
Patient and public involvement: Patients or the public were not involved in the design, conduct, reporting or dissemination plans of our research.
Data sharing: Requests for the E-CATCO cost-effectiveness analysis and any further data requests can be made to the corresponding author.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/10/3/E807/suppl/DC1.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- © 2022 CMA Impact Inc. or its licensors