Abstract
Background: Dual antiplatelet therapy (DAPT) is routinely given to patients after percutaneous coronary intervention (PCI) with stenting; however, optimal duration remains uncertain in some situations. We assessed the benefits and harms of extending DAPT beyond 1 year after PCI in clinically important patient subgroups.
Methods: We conducted a systematic review and meta-analysis. We searched electronic databases (Embase, MEDLINE, PubMed, Cochrane Library) and grey literature (from inception to Nov. 5, 2021) and included randomized controlled trials (RCTs) of extended DAPT (> 12 mo) compared with DAPT for 6–12 months following PCI with stenting. The primary outcome was death (all cause, cardiovascular, noncardiovascular); secondary outcomes included major adverse cardiovascular and cerebrovascular events, myocardial infarction (MI), stroke, stent thrombosis and bleeding. Subgroups were based on prespecified patient characteristics (prior MI, acute coronary syndrome [ACS], diabetes mellitus, age, smoking status). Data were analyzed by random-effects pairwise meta-analysis.
Results: We identified 9 RCTs that provided subgroup data. We found that extended DAPT reduced the risk of MI and stent thrombosis but increased the risk of bleeding, compared with standard DAPT, with no difference in the risk of all-cause death (relative risk [RR] 1.07, 95% confidence interval [CI] 0.80–1.42) or cardiovascular death (RR 0.98, 95% CI 0.74–1.30). We found that patients with a prior MI, with ACS at presentation, without diabetes or aged younger than 75 years may derive the most benefit from extended DAPT. Among patients who received extended DAPT, the risk of all-cause death was significantly increased among those with no prior MI (RR 1.64, 95% CI 1.08–2.24), whereas there was no significant difference in the risk of all-cause death between standard and extended DAPT for patients with ACS (RR 1.20, 95% CI 0.51–2.83), with diabetes (RR 1.27, 95% CI 0.86–1.89), aged older than 75 years (RR 1.32, 95% CI 0.39–4.54) or who smoked (RR 0.90, 95% CI 0.42–1.92). Similar results were found for cardiovascular death, where data were available.
Interpretation: Patients with a previous MI with ACS at presentation, without diabetes, or aged younger than 75 years may derive the most benefit from extended DAPT. These findings support the need for careful selection of patients who may benefit most from extended DAPT.
Study registration: PROSPERO no. CRD42018082587
After percutaneous coronary intervention (PCI) with implantation of drug-eluting or bare-metal stents, patients are given dual antiplatelet therapy (DAPT; P2Y12 inhibitor plus acetylsalicylic acid) with the goal of preventing stent thrombosis and other major adverse cardiac and cerebrovascular events (MACCEs). The optimal duration of DAPT remains uncertain in some situations,1 and patient characteristics may be important in determining the optimal duration.2 For some, DAPT for 6–12 months may be appropriate after stenting, whereas others may derive greater benefit from extending DAPT beyond 12 months. Guidelines by the American College of Cardiology–American Heart Association,3 European Society of Cardiology4 and the Canadian Cardiovascular Society5 recommend that extended DAPT be considered for patients at high risk of thrombotic events and low risk of bleeding.
Several randomized controlled trials (RCTs) have assessed the effect of extending DAPT beyond 12 months.6–11 Previous meta-analyses have provided estimates of the overall relative effect of extended compared with shorter-duration DAPT,12–22 finding that extended DAPT reduces the risk of myocardial infarction (MI) and stent thrombosis, but increases the bleeding risk.1 However, these meta-analyses have typically pooled all trial participants, despite the importance of individual patient characteristics in the decision to extend DAPT.23 Therefore, there remains an uncertainty about the relative benefits and harms of extended DAPT among patient subgroups.
To address this gap, we performed a systematic review and meta-analysis of RCTs to assess the relative benefits and harms of extended DAPT (> 12 mo), compared with standard DAPT (6–12 mo), after PCI with stenting in clinically important patient subgroups, including those with previous MI, acute coronary syndrome (ACS) or diabetes, as well as by age and smoking status.
Methods
We undertook a systematic review using the methods of the Cochrane Handbook for Systematic Reviews for Interventions, with reporting guided by the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) checklist for systematic reviews.24 The review protocol was registered in PROSPERO (CRD42018082587) and published.25
Study selection and search strategy
We based our study searching and selection on the Population, Intervention, Comparison, Outcomes and Study (PICOS) design criteria, described in detail in Box 1. An experienced information specialist (B.S.) developed the search strategy in consultation with the review team. Using the Peer Review of Electronic Search Strategies (PRESS) Checklist,26 the MEDLINE strategy was peer reviewed by another senior information specialist before translation to the other databases. We searched MEDLINE (1947–present) and Embase (1974–present) in multifile on Ovid, the Cochrane Central Register of Controlled Trials (CENTRAL) database of the Cochrane Library (Wiley version) and PubMed from inception to Nov. 5, 2021 (i.e., 2021, Issue 10 of CENTRAL). There were no date or language restrictions on any of the searches. The search strategy used controlled vocabulary appropriate to each database (e.g., MEDLINE medical subject headings “stents,” “percutaneous coronary intervention,” “purinergic P2Y receptor antagonists”) and keywords (e.g., “DES,” “PCI,” “dual antiplatelet therapy”) (Appendix 1, available at www.cmajopen.ca/content/11/1/E118/suppl/DC1). We searched ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP) for ongoing and completed clinical trials. Results were downloaded and deduplicated in Reference Manager (version 12) and uploaded to Distiller SR.
Population, Intervention, Comparison, Outcomes and Study (PICOS) design criteria
Population: Adults (≥ 18 yr) who had undergone percutaneous coronary intervention (PCI) with a bare-metal or drug-eluting stent.
Intervention: Dual antiplatelet therapy (DAPT; ≥ 12 mo) following PCI with stenting (extended DAPT).
Comparator: Dual antiplatelet therapy (6–12 mo) following PCI with stenting (standard DAPT) which may involve any type of P2Y12 inhibitor (e.g., clopidogrel, prasugrel, ticagrelor) in combination with acetylsalicylic acid.
Outcomes: The primary outcome was death (all-cause, cardiovascular, noncardiovascular). Secondary outcomes were major adverse cardiac and cerebrovascular event, myocardial infarction (MI), stroke, stent thrombosis, bleeding and urgent target vessel revascularization.
Study design: Randomized controlled trials.
Subgroups of interest: Patients with previous MI, acute coronary syndrome at presentation, type 2 diabetes mellitus (diabetes), age (< 75 yr, ≥ 75 yr) and smoking status.
Studies were selected in duplicate by independent reviewers (J.E., Z.B., C.L.) based on title and abstract screening of each identified record. The full text of all abstracts deemed potentially relevant was evaluated for eligibility (J.E., Z.B.), and any disagreements were resolved through discussion among reviewers or in consultation with an additional author (S.E.K.). Study selection was guided by the PICOS criteria (Box 1).
Data extraction
Data were extracted by a reviewer and checked by a second reviewer (J.E., Z.B.). Data were extracted from the original, primary publication for each included RCT, with supplementary data obtained from companion reports or clinical trial registration records, all of which were identified as part of database and grey literature searches. Data were extracted based on patient characteristics (prior MI, ACS at presentation, diabetes, age, smoking status). Disagreements were resolved by discussion.
Risk of bias
Two independent reviewers (J.E., Z.B.) assessed the risk of bias using the Cochrane Collaboration’s risk of bias tool,27 with consensus reached through discussion. Additional information was sought from companion publications (e.g., supplements, clinical trial registries, post-hoc analyses). Publication bias was assessed for outcomes with data from at least 10 studies.27
Statistical analysis
We present a descriptive summary for study selection, quality assessment and study and patient characteristics. Clinical heterogeneity was assessed by examining participant characteristics. Methodological heterogeneity was assessed by examination of study design characteristics, and data were pooled from studies deemed methodologically similar. Statistical heterogeneity was assessed by use of the I2 statistic with I2 greater than 75% considered to represent substantial statistical heterogeneity, and pooled data were not reported above this threshold. Data were analyzed by random-effects pair-wise meta-analysis, with relative risks (RRs) and hazard ratios (HRs) (with 95% confidence intervals [CIs]) presented. Where available, the number of participants randomized was used as the denominator, whereas the number of participants who experienced each outcome formed the numerator; otherwise, group-level data (RR or HR) are presented, with CIs, as reported in the publication. Bleeding outcomes were analyzed separately by classification type or definition (e.g., Thrombolysis in MI [TIMI], Bleeding Academic Research Consortium [BARC], Global Use of Strategies to Open Occluded Coronary Arteries [GUSTO] classification systems). 28 For MACCE, data were pooled only for studies that used a comparable definition (i.e., including all-cause death, MI, stroke). Although we had intended to perform network meta-analysis to analyze the effects of individual P2Y12 inhibitors, there were insufficient subgroup data to permit such analyses. More details of the analysis plan can be found in the protocol.25
Ethics approval
Our systematic review and meta-analysis used publicly available aggregate data; as such, ethics approval was not required.
Results
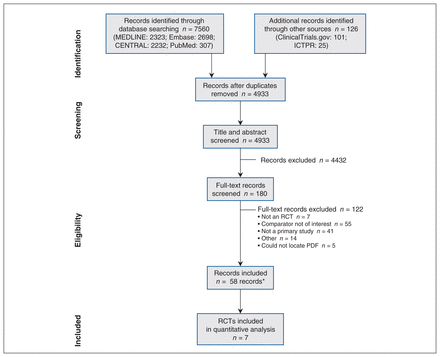
The initial search identified 7506 records (Figure 1), with an additional 126 records identified by grey literature searching. Among these, 180 records were examined in full text, with 58 meeting the PICOS criteria (Appendix 2, available at www.cmajopen.ca/content/11/1/E118/suppl/DC1). Sixteen RCTs were included, corresponding to 9 unique RCTs reporting data6–11,29–31 and an additional 7 RCTs32–38 with no outcome data (e.g., ClinicalTrials.gov record without results).
PRISMA flowchart of study selection. *Fifty-eight records corresponded to 16 unique randomized controlled trials (RCTs), of which 9 reported outcome data. Owing to differences in the timing of randomization, 2 RCTs were excluded from quantitative data analyses. Note: CENTRAL = Cochrane Central Register of Controlled Trials, ICTPR = International Clinical Trials Registry Platform, PRISMA = Preferred Reporting Items for Systematic reviews and Meta-Analyses.
Among the 9 RCTs reporting data, 4 RCTs6,8,9,11 randomized participants after completion of at least 12 months (12–18 mo) of DAPT without an adverse event, which excluded participants at high risk of an adverse event immediately after stenting, whereas 5 RCTs7,10,29–31 randomized participants within the first 30 days after stenting. To ensure consistency across studies, we included 6-month landmark data from the Nobori Dual Antiplatelet Therapy as Appropriate Duration (NIPPON) trial,31 Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study (PRODIGY) trial,10 and the Is There a Life for DES After Discontinuation of Clopidogrel (ITALIC) trial7 along with data from trials that randomized participants after at least 6 months of DAPT; thus, the evidence base for this review is formed by 7 RCTs6–11,31 (Table 1).
Characteristics of included randomized controlled trials
Trial and participant characteristics
The included RCTs were published between 2012 and 2017, and involved 1286–11 648 participants (Table 1). In total, 25 982 participants were randomized, with 13 041 receiving extended DAPT and 12 941 receiving DAPT. Three RCTs7,10,31 compared extended DAPT with 6 months of DAPT, whereas 4 compared extended DAPT with 12 months of DAPT.6,8,9,11 Most RCTs involved drug-eluting stents, with the exception of the DAPT and PRODIGY trials, in which 15% to 25% of participants received a bare-metal stent. Clopidogrel was the most frequently used P2Y12 inhibitor, with exclusive use in 3 RCTs (Appendix 3, available at www.cmajopen.ca/content/11/1/E118/suppl/DC1).8–10
The mean age of the included participants was 60 years or older (Appendix 4, available at www.cmajopen.ca/content/11/1/E118/suppl/DC1). Most participants were male (69%–82%) and smoking was common (23%–61%). The prevalence of diabetes ranged from 24% to 38%, and between 4% and 31% of participants had a previous MI. There was a wide variation in the prevalence of ACS (0.1%–33% had ST-elevation MI [STEMI]), 2%–23% had non-STEMI and 9%–39% had unstable angina (Appendix 5, available at www.cmajopen.ca/content/11/1/E118/suppl/DC1). These differences may be due, in part, to the eligibility criteria of each RCT (Appendix 6, available at www.cmajopen.ca/content/11/1/E118/suppl/DC1).
Risk of bias assessment
Overall, the RCTs were judged to be at low risk of bias across all domains (Appendix 7, available at www.cmajopen.ca/content/11/1/E118/suppl/DC1). Although all included RCTs employed an open-label design, knowledge of treatment assignment would not be expected to have a substantive effect on the study outcomes. Three RCTs7,8,31 were at an unclear risk of other sources of bias because of early termination. Publication bias could not be formally assessed for any outcome as less than 10 RCTs were included for all outcomes.
Outcomes
All participants
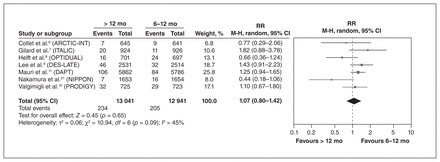
Compared with standard DAPT, extended DAPT reduced the risk of MI (RR 0.58, 95% CI 0.48–0.70) and probable or definite stent thrombosis (RR 0.38, 95% CI 0.21–0.67) (Table 2). The risk of moderate bleeding was generally higher, although findings differed by classification system (Table 2). There were no significant differences for all-cause death (Figure 2) (RR 1.07, 95% CI 0.80–1.42) or cardiovascular death (RR 0.98, 95% CI 0.74–1.30), stroke, definite stent thrombosis, urgent revascularization or MACCE (see Table 2 for all estimates). Findings related to the risk of noncardiovascular death were inconsistent across RCTs and were not pooled because of high heterogeneity. The DAPT trial11 reported an increased risk of noncardiovascular death among patients who received extended DAPT, although this finding was not rep-licated in 2 smaller RCTs.8,31
Benefits and harms of extended dual antiplatelet therapy (DAPT; > 12 mo) compared with standard DAPT (6–12 mo) among all trial participants*
Benefits and harms of extended dual antiplatelet therapy (DAPT) (> 12 mo) compared with standard DAPT (6–12 mo) among all trial participants. Note: CI = confidence interval, M-H = Mantel–Haenszel, RR = relative risk.
Clinically important patient subgroups
Prior MI
Two RCTs7,11 reported outcome data based on participants’ history of MI (Table 3). The DAPT trial11 reported on participants with a previous MI, prior MI (> 72 h before PCI), index MI (within 72 h of PCI) and both prior and index MI. For consistency, data for any MI from the DAPT trial were pooled with data for participants with a history of MI as reported in the ITALIC trial.
Benefits and harms of extended dual antiplatelet therapy (DAPT; > 12 mo) compared with standard DAPT (6–12 mo) among participants with or without prior myocardial infarction*
Among participants with a previous MI, extended DAPT was associated with a lower risk of MI (RR 0.48, 95% CI 0.36–0.64), probable or definite stent thrombosis (RR 0.29, 95% CI 0.16–0.52) and MACCE (RR 0.67, 95% CI 0.53–0.83), but a higher risk of GUSTO moderate bleeding (RR 2.30, 95% CI 1.28–4.11), GUSTO moderate or severe bleeding (RR 1.89, 95% CI 1.21–2.95) or BARC type 2, 3, 5 bleeding (RR 2.06, 95% CI 1.50–2.82). There were no significant differences for all-cause death (RR 1.04, 95% CI 0.72–1.51) or cardiovascular death (RR 0.52, 95% CI 0.05–5.69), stroke, urgent revascularization or other bleeding outcomes between extended and standard DAPT.
Among participants with no previous MI, data from the DAPT trial suggest that extended DAPT is associated with a lower risk of MI (RR 0.63, 95% CI 0.46–0.87) and probable or definite stent thrombosis (RR 0.32, 95% CI 0.15–0.68), and a higher risk of bleeding (GUSTO moderate, GUSTO moderate or severe, BARC type 2, 3, 5 bleeding; Table 3). In contrast to patients with prior MI, the risk of all-cause death was significantly increased among participants with no prior MI who received extended DAPT (RR 1.64, 95% CI 1.08–2.48). There were no other significant differences (Table 3).
Acute coronary syndrome at presentation
Three RCTs7,9,11 reported data for participants with ACS (Table 4). Among these, 2 trials7,9 categorized participants as having ACS or no ACS, whereas 1 RCT reported ACS data for participants with an index MI (occurring < 72 h before the index PCI).11 Among participants with ACS, extended DAPT was associated with a lower risk of MI (RR 0.49, 95% CI 0.29–0.85), probable or definite stent thrombosis (RR 0.26, 95% CI 0.12–0.54), but a higher risk of bleeding (GUSTO moderate; GUSTO moderate or severe; BARC type 2, 3, 5; Table 4). One RCT11 reported a significant reduction in MACCE with extended DAPT (RR 0.57, 95% CI 0.43–0.76); however, this finding was not replicated in a second RCT7 and high statistical heterogeneity precluded pooling. There were no other significant differences (Table 4), including for all-cause death (RR 1.20, 95% CI 0.51–2.83) or cardiovascular death (RR 0.66, 95% CI 0.11–3.91).
Benefits and harms of extended dual antiplatelet therapy (DAPT; > 12 mo) compared with standard DAPT (6–12 mo) among participants with or without acute coronary syndrome at presentation*
Data were limited among participants without ACS. One RCT9 reported no significant difference in MACCE between extended and shorter DAPT (RR 1.14, 95% CI 0.67–1.95); no data were available for other outcomes.
Diabetes
Three RCTs7,11,15 reported data for participants with or without diabetes (Table 5). Among participants with diabetes, there was no significant difference in the risk of death (all-cause RR 1.27, 95% CI 0.86–1.89; cardiovascular RR 1.02, 95% CI 0.61–1.71; noncardiovascular RR 1.71, 95% CI 0.79–3.70), MI (RR 0.74, 95% CI 0.54–1.02), stroke (RR 1.01, 95% CI 0.52–1.95), probable or definite stent thrombosis (RR 0.48, 95% CI 0.21–1.06) and urgent revascularization (RR 0.96, 95% CI 0.20–4.74) between extended and standard DAPT. Two RCTs10,11 reported MACCE, although the use of different outcome measures (RR, HR) precluded pooling of the data; however, both reported no significant difference in the risk of MACCE between standard and extended DAPT.
Benefits and harms of extended dual antiplatelet therapy (DAPT) compared with DAPT for 6–12 months among participants with or without diabetes*
Among participants without diabetes, there was no significant difference in all-cause death (RR 1.24, 95% CI 0.86–1.80) (Table 5), although a lower risk of MI (RR 0.44, 95% CI 0.33–0.59) and stent thrombosis (RR 0.29, 95% CI 0.17–0.50) was reported among participants without diabetes who received extended DAPT. Inconsistent findings were noted for the risk of MACCE as 1 RCT10 reported no significant difference in MACCE (HR 1.06, 95% CI 0.76–1.50), whereas 1 RCT11 reported a significantly lower risk of MACCE (RR 0.63, 95% CI 0.51–0.78), for a patient who received extended DAPT. No studies assessed cardiovascular or noncardiovascular death, stroke or urgent revascularization.
Age group
Three RCTs7,10,11 reported outcome data by age group (≥ 75 yr, < 75 yr; Table 6). Among participants aged older than 75 years, a single RCT10 reported an increased risk of stroke among those who received extended DAPT (RR 8.59, 95% CI 1.08–68.28). The risk of bleeding was also increased in this subgroup (GUSTO moderate to severe, RR 5.01, 95% CI 1.46–17.26); however, this finding was not replicated in the larger DAPT trial11 (HR 1.03, 95% CI 0.54–1.98). There were no significant differences in the risks of all-cause death (RR 1.32, 95% CI 0.39–4.54) or cardiovascular death (RR 0.98, 95% CI 0.24–4.04), MI, stent thrombosis, urgent revascularization, MACCE or minor bleeding between extended and standard DAPT among participants aged 75 years and older (Table 6).
Benefits and harms of extended dual antiplatelet therapy (DAPT; > 12 mo) compared with standard DAPT (6–12 mo) among participants aged younger than 75 years or 75 years and older*
Two RCTs reported outcomes for participants aged younger than 75 years.10,11 Owing to reporting differences (i.e., HR v. RR), the findings reported by the DAPT and PRODIGY trials could not be pooled, and are presented separately in Table 6. The DAPT trial11 reported a reduction in the risk of MI, probable and stent thrombosis and MACCE among participants aged younger than 75 years. These findings were not replicated in the smaller PRODIGY trial.10
Smoking
Limited subgroup data were available by smoking status. In total, 3 RCTs6,10,11 reported outcome data by smoking status. One RCT10 categorized participants as smokers or nonsmokers, 1 RCT11 categorized smoking status as current tobacco use and no current tobacco use, and 1 RCT6 categorized smoking as current smoking and no smoking. For this analysis, we considered smoking, current tobacco use, and current smoking to include participants who smoke.
Among smokers and nonsmokers, extended DAPT significantly decreased the risk of MI and probable or definite stent thrombosis (Table 7). Differential effects between subgroups were noted for MACCE (significantly reduced among smokers) and bleeding (increased among nonsmokers) with extended DAPT. One additional RCT6 assessed MACCE among smokers and nonsmokers using an alternative definition (including all-cause death, MI, stent thrombosis, stroke, urgent revascularization), finding a non-significant difference in risk between DAPT durations for both smokers (RR 0.86, 95% CI 0.27–2.76) and nonsmokers (RR 0.88, 95% CI 0.48–1.61).
Benefits and harms of extended dual antiplatelet therapy (DAPT; > 12 mo) compared with standard DAPT (6–12 mo), by smoking status*
Interpretation
Dual antiplatelet therapy is required after coronary revascularization; however, the optimal duration of treatment requires balancing the potential benefits and harms, which may depend on individual patient characteristics. In this systematic review of clinically important patient subgroups, we found that patients with a prior MI, with ACS at presentation, without diabetes, or aged younger than 75 years, may derive the most benefit from extended DAPT. The findings of this review support individualizing DAPT based on patient-specific risk factors.
Many systematic reviews have attempted to elucidate the optimal duration of extended DAPT after PCI with stenting; however, few have taken individual patient characteristics into account,1 despite guideline recommendations to tailor the duration of DAPT to patient characteristics.3–5 To address this evidence gap, we undertook a systematic review to address the question of the optimal duration of DAPT among such subgroups. Our review includes the same core set of RCTs included in most previous systematic reviews (PRODIGY trial,10 Drug-Eluting Stents to Reduce Late Coronary Arterial Thrombotic Event [DES-LATE] trial,9 Assessment by a Double Randomization of a Conventional Antiplatelet Strategy Versus a Monitoring-Guided Strategy for Drug-Eluting Stent Implantation and, of Treatment Interruption Versus Continuation One Year After Stenting [ARCTIC-Interruption] trial,6 ITALIC trial,7 DAPT trial,11 OPTImal DUAL Antiplatelet Therapy [OPTIDUAL] trial8). We used companion trial reports and additional analyses to provide data for these subgroups. Our findings, when not stratified by subgroup, are consistent with previous reviews that have reported a lower risk of MI and stent thrombosis with extended DAPT, compared with standard DAPT; however, we identified important differences in outcomes based on patient characteristics.
This review also serves to identify important gaps for future research. Notably, there was little available evidence for some clinically important subgroups (e.g., by smoking status, age group). Although the question of the optimal duration of DAPT treatment is not new, relatively few trials have assessed the continuation of DAPT beyond 12 months after stenting, and the majority of the evidence for most subgroups is from the DAPT trial, the largest RCT to assess the question of the optimal duration of DAPT. However, important differences have been noted between the findings of the DAPT trial and other trials, including an increased risk of all-cause death in the DAPT trial that was not replicated in other studies. We noted other such differences in findings between the DAPT trial and other RCTs, including the risk of MI, stent thrombosis and MACCE among patients aged younger than 75 years and those without diabetes.
The importance of patient characteristics has long been recognized in determining the optimal duration of DAPT. The DAPT score,23 a prediction tool for estimating the benefits and harms of extending DAPT for more than 12 months after PCI, incorporates many of the characteristics considered in this review, including a history of MI, diabetes, age and smoking. The original DAPT score showed modest accuracy in predicting which patients would be at higher risk of late ischemic and bleeding events with extended DAPT,23 whereas subsequent studies have shown that the DAPT score is a more accurate predictor of benefits and harms among patients with a prior MI, owing to a higher risk of adverse outcomes in this group. This further highlights the importance of considering individual patient characteristics in the decision to extend DAPT.39
Limitations
This review has several limitations that merit consideration. All included trials were open label. However, knowledge of treatment assignment would not be expected to have a substantive effect on the effect estimates for the outcomes of interest. Initial randomization may not hold in subgroups, potentially leading to imbalances between treatment groups, and we did not formally assess effect modification by patient characteristics. Further, the findings may not be generalizable to all patients in clinical practice, as some high-risk patients may have been excluded based on trial eligibility or owing to randomization after the completion of an initial event-free period in most trials. Outcome definitions for MACCE and major bleeding varied across trials. We reported data separately that were assessed by using different bleeding classification scales, and, for MACCE, we pooled only data from trials that used a comparable definition of the composite outcome, to increase homogeneity. Limited data were available for some patient subgroups, limiting the power of these analyses to detect differences between DAPT durations and increasing the probability of a false-negative finding. Finally, given the large number of comparisons and limited power for some, we cannot exclude the possibility of type I and type II errors.
Conclusion
Individual patient characteristics are important in determining the benefits and harms of extending DAPT beyond 12 months after stenting. Patients with prior MI and those with ACS at presentation, as well as patients without diabetes or aged younger than 75 years, may derive the most benefit from extended DAPT provided that the increased risk of bleeding is accounted for. These findings support the need for the careful selection of patients who may benefit most from extending the duration of DAPT beyond 12 months.
Acknowledgement
The authors thank Caroline Eagles for assistance with study selection.
Footnotes
Competing interests: Derek So has received unrestricted grants from Eli Lilly Canada and Spartan Biosciences for physician-initiated studies and has served as an advisory board member for AstraZeneca Canada. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Jesse Elliott, Shannon Kelly, Zemin Bai, Michel Boucher, Derek So and George Wells contributed to the conception and design of the work. Becky Skidmore designed and executed the search strategy. Jesse Elliott and Zemin Bai contributed to the acquisition and analysis of the data. Jesse Elliott, Shannon Kelly, Derek So, Michel Boucher and George Wells interpreted the data. Jesse Elliott drafted the manuscript. All authors revised the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This research was funded by the Canadian Agency for Drugs and Technologies in Health (Ottawa, Ontario), an independent organization established and funded by the federal, provincial and territorial governments in Canada.
Disclaimer: Jesse Elliott is an associate editor for CMAJ Open and was not involved in the editorial decision-making process for this article.
Data sharing: Data are available from the corresponding author by way of email.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/11/1/E118/suppl/DC1.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use) and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/
References
- © 2023 CMA Impact Inc. or its licensors