Abstract
Background: Accurate and timely testing for SARS-CoV-2 in the pediatric population is crucial to control the COVID-19 pandemic; saliva testing has been proposed as a less invasive alternative to nasopharyngeal swabs. We sought to compare the detection of SARS-CoV-2 using saliva versus nasopharyngeal swab in the pediatric population, and to determine the optimum time of testing for SARS-CoV-2 using saliva.
Methods: We conducted a longitudinal diagnostic study in Ottawa, Canada, from Jan. 19 to Mar. 26, 2021. Children aged 3–17 years were eligible if they exhibited symptoms of COVID-19, had been identified as a high-risk or close contact to someone confirmed positive for SARS-CoV-2 or had travelled outside Canada in the previous 14 days. Participants provided both nasopharyngeal swab and saliva samples. Saliva was collected using a self-collection kit (DNA Genotek, OM-505) or a sponge-based kit (DNA Genotek, ORE-100) if they could not provide a saliva sample into a tube.
Results: Among 1580 paired nasopharyngeal and saliva tests, 60 paired samples were positive for SARS-CoV-2. Forty-four (73.3%) were concordant-positive results and 16 (26.6%) were discordant, among which 8 were positive only on nasopharyngeal swab and 8 were positive only on saliva testing. The sensitivity of saliva was 84.6% (95% confidence interval 71.9%–93.1%).
Interpretation: Salivary testing for SARS-CoV-2 in the pediatric population is less invasive and shows similar detection of SARS-CoV-2 to nasopharyngeal swabs. It may therefore provide a feasible alternative for diagnosis of SARS-CoV-2 infection in children.
Diagnostic testing for SARS-CoV-2 is crucial in the management of the COVID-19 pandemic. According to the Public Health Agency of Canada (PHAC), nasopharyngeal swabs are the specimen of choice for routine testing for SARS-CoV-2.1 Nasopharyngeal swabbing is an uncomfortable procedure, which may decrease patient willingness to comply with repeat testing, and increase use of health care personnel, costs and exposure risk.2,3 The need for mass testing also creates bottlenecks in sample collection and depletes availability of protective equipment. Therefore, alternative testing methods are needed.
Salivary testing has been proposed as an alternative to nasopharyngeal swabs. We previously compared detection rates of nasopharyngeal swabs with self-collection saliva kits for SARS-CoV-2 in adults.4 However, limited literature is available to support salivary testing in the pediatric population, and those published show conflicting results. Select studies show similar detection between the 2 approaches, with a high sensitivity of saliva when using nasopharyngeal as a reference standard.2,5–7 In contrast, other studies found lower rates, but were limited by small sample sizes.8,9 Furthermore, no studies to date have evaluated the optimum timing for testing after exposure or symptom onset in the adult or pediatric populations. Previous studies have shown variability of results produced at different time points.10 New studies are needed to determine the optimum timing for testing for these diagnostic modalities.
To date, children younger than 19 years account for about 20% of SARS-CoV-2 infections in Canada.11 As such, the need for effective and reliable repeat sampling in children is critical as restrictions ease and the prevalence of variants of concern increases, especially for distinguishing SARS-CoV-2 infection from other respiratory pathogens in children. As a less invasive testing method, saliva testing may prove an effective alternative to repeat testing by nasopharyngeal swab in the context of frequent testing in the pediatric population. In this study, our primary objective was to compare the detection of SARS-CoV-2 using a self-administered kit for saliva collection with using nasopharyngeal swabs in the pediatric population. Our secondary objective was to determine the optimal time for salivary testing to achieve maximum sensitivity.
Methods
Study design and setting
In this longitudinal diagnostic study, all patients presenting to the regional COVID-19 Pediatric Assessment Centre in Ottawa between Jan. 19 and Mar. 26, 2021, were eligible for enrolment (Appendix 1, Supplementary Appendix A, available at www.cmajopen.ca/content/10/4/E981/suppl/DC1).
Study participants
Children aged 3–17 years were eligible to participate if they were either identified as having high-risk or close contact to someone confirmed to be positive for SARS-CoV-2,12 had travelled outside Canada within 14 days or exhibited symptoms of COVID-19 (Appendix 1, Supplementary Appendix A, Table 1). Children were ineligible if they or their primary caregiver were unable or unwilling to perform the saliva test or complete the at-home portion of the testing, or were unable to communicate in English or French, preventing informed consent. Children were excluded if symptom onset began more than 2 months before testing (Appendix 1, Supplementary Appendix A).
Participant baseline characteristics
sStudy procedures
We collected baseline demographics and indication for testing from all study participants. All study participants provided 1 saliva specimen concurrent with their standard nasopharyngeal swab test. Participants were then sent home with 2 saliva self-collection kits, and were required to provide saliva samples at home on the third and seventh day after initial collection. We selected the third and seventh days as viral load is anticipated to peak around these dates. Children aged 6–17 years used a saliva self-collection kit (DNA Genotek, OM-505),13 for which participants were required to produce 1 mL of saliva into a tube. Children aged 3–5 years, and those unable to provide a saliva sample owing to physical or mental limitations used a sponge-based kit (DNA Genotek, ORE-100),14 which involved inserting a small sponge on a stick into the child’s mouth in the area between the teeth and the cheek for about 1 minute. The results from these 2 kits are expected to be similar.13,14 Self-collected samples were obtained at least 30 minutes after abstinence from food or drink.5,6 These kits are designed for self-or parent-supported collection without expert assistance and can preserve viral material at room temperature for transport and analysis for up to 3 weeks after collection.13,14 Saliva samples were collected according to the manufacturer recommendations (Appendix 1, Supplementary Appendix B).
Outcomes and measures
All nasopharyngeal swabs were collected at the COVID-19 Assessment Centre and sent to the Eastern Ontario Regional Laboratory Association for RNA extraction and analysis by polymerase chain reaction (PCR). Saliva samples collected from the Assessment Centre were shipped to the National Microbiology Laboratory (NML), the centralized laboratory of the PHAC, in Winnipeg, Manitoba. Samples collected at participants’ homes were mailed first to the research team and subsequently shipped to NML. At NML, saliva samples underwent nucleic acid extraction and real-time reverse transcription quantitative PCR (RT-qPCR) for SARS-CoV-2. We extracted nucleic acid in saliva using the MagMAX-96 Viral RNA Isolation Kit (Thermo Fisher Scientific) as per the manufacturer’s protocol. Extraction took place on a KingFisher Flex device (Thermo Fisher Scientific). We used TaqPath 1-Step RT-qPCR Master Mix (Thermo Fisher Scientific) for PCR on extracted saliva RNA samples. After extraction, we conducted RT-qPCR, targeting the E gene with confirmation with the RdRp gene. The limit of detection for both genes were 2.6−5 TCID50 (median tissue culture infectious dose).15,16
We defined a saliva sample as positive if both the E and RdRp genes produced cycle threshold17 values less than 40. We considered any cycle threshold values of 40 or greater a negative result for that gene target. If neither the E gene nor RdRp gene produced a cycle threshold value less than 40, we considered this to be a negative result. If only 1 of the 2 gene targets produced a cycle threshold value less than 40, we re-extracted and re-ran the sample. If both targets produced cycle threshold values less than 40 on the re-run, we considered this to be positive. If the same 1 gene target produced a cycle threshold value less than 40 and the other did not (i.e., the sample replicated the same results from the first extraction), we considered this to be a positive result. If a re-extracted sample did not match 1 of the 2 aforementioned possibilities, then we considered the overall result of the sample to be indeterminate. Various assays were used for nasopharyngeal swab analysis, and not all assays quantified the cycle threshold values. We considered all positive results, either on saliva or swab, as true positive results for analysis purposes.
Statistical analysis
We used demographic data to inform the descriptive analysis, and excluded missing data from the analysis. We classified participants as having concordant test results if both nasopharyngeal swab and saliva-based assay results were the same, and otherwise considered them discordant. We used the Cohen κ statistic, a measure used to assess the level of agreement between 2 tests beyond chance,18 and the prevalence-adjusted κ (PABAK) statistic,19 an adapted measure to account for low prevalence (Appendix 1, Supplementary Appendix A, Equations), to quantify agreement between nasopharyngeal swab and saliva tests.18–20 We computed the probability of being asymptomatic or symptomatic while having either concordant or discordant results (Appendix 1, Supplementary Appendix A, Equations). We visualized all cycle threshold values on scatter plots to identify trends.
Lastly, we simulated a testing schedule of 30 days, with 3 scenarios whereby testing is performed every 2, 5 or 7 days. This was done to assess and compare the hypothetical performance of repeat testing using nasopharyngeal- or saliva-based samples (Appendix 1, Supplementary Appendix C).
We performed statistical analysis in R version 4.1.1.
Ethics approval
This study was approved by the Children’s Hospital of Eastern Ontario (CHEO) Research Ethics Board (#21/04X), Ottawa Health Science Network Research Ethics Board and the PHAC Research Ethics Board.
Results
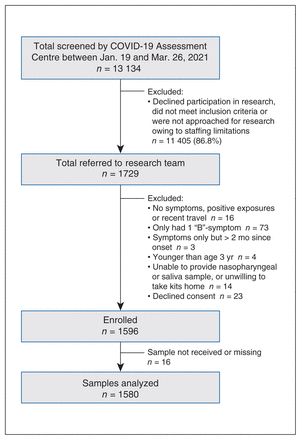
Among 13 134 children tested at the COVID-19 Assessment Centre between Jan. 19 and Mar. 26, 2021, 1596 participants consented and were enrolled in our study (Figure 1). Among these participants, 1580 successfully provided both a nasopharyngeal swab and saliva sample at the COVID-19 Assessment Centre.
Study flowchart. “B” symptoms include congestion, sore throat, abdominal pain, vomiting, diarrhea, fatigue, loss of appetite, generalized muscle pain and headache (Appendix 1, Supplementary Appendix A, available at www.cmajopen.ca/content/10/4/E981/suppl/DC1).
Descriptive results
Demographics can be found in Table 1. The average test positivity in Ottawa was 2.5%–7.5% during the study period.21 Among the 1580 paired samples received at the first visit, 60 tested positive for SARS-CoV-2. Of these, 44 (73.3%) tested positive on both saliva and swab tests, 8 tested positive only on nasopharyngeal swab and 8 tested positive only on saliva; 1520 tested negative on both test modalities (Table 2). We found an overall concordance rate of 99.0% (95% confidence interval [CI] 98.3%–99.4%).
Contingency table of concordant and discordant test results
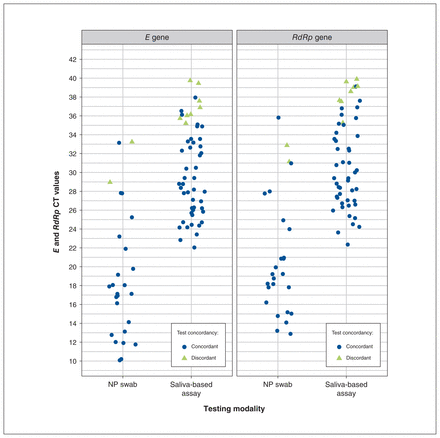
We found high agreement between nasopharyngeal swab and saliva tests, with a Cohen κ value of 0.84 (95% CI 0.76–0.92) and PABAK value of 0.98 (95% CI 0.97–0.99). Using nasopharyngeal swabs as the reference group, the sensitivity of saliva assays at the baseline visit was 84.6% (95% CI 71.9%–93.1%), with a specificity of 99.5% (95% CI 99.0%–99.8%). The probability of being asymptomatic and receiving discordant results (2%) was 4 times that of being symptomatic and receiving discordant results (0.5%) (Appendix 1, Supplementary Appendix D, Table 1). Cycle threshold values were available for all saliva samples, and for 26 of 52 individuals who tested positive on nasopharyngeal swab. Individuals with discordant test results tended to have higher cycle threshold values (Figure 2).
Trends in cycle threshold (CT) values by target gene, stratified by test concordancy, for positive tests. Note: NP = nasopharyngeal.
Longitudinal analysis
Overall, 582 saliva-based samples were collected within 1 day of symptom onset, 566 were collected 3–4 days after symptoms onset and 496 were collected 7–8 days after symptoms onset (Appendix 1, Supplementary Appendix D, Figure 1). Of the 1580 participants who successfully provided saliva samples, 42 had at least 2 cycle threshold values measured at any of the 3 collected saliva samples, totalling 112 cycle threshold measurements. The average difference in cycle threshold values between the last and first samples was 2.9 (median 3.4, interquartile range −0.5 to 6.4); 73.8% had a positive difference, indicating an increasing trend in cycle threshold values among participants over time (Appendix 1, Supplementary Appendix D, Figure 2).
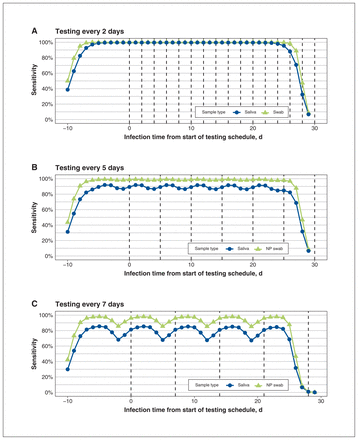
Our simulation suggests that a 30-day testing schedule with repeat testing every 2 days (using an assay based on nasopharyngeal swab or saliva) can reach sensitivity levels close to 100% (Figure 3). However, as testing frequency decreases to 5 days, the mean overall sensitivity for the saliva-based assay is 89.2% (95% CI 86.4%–91.8%), lower than for the nasopharyngeal swab (98.6%, 95% CI 97.9%–99.1%). If testing every 7 days, the mean overall sensitivity of the saliva-based assay drops to 79.0% (95% CI 67.4%–85.3%) (Figure 3; Appendix 1, Supplementary Appendix C).
Simulation of repeat saliva testing for SARS-CoV-2 every (A) 2, (B) 5 and (C) 7 days (vertical dashed lines). Note: NP = nasopharyngeal. Model assumptions for this simulation and detailed explanation of the interpretation of the figure are described in Appendix 1, Supplementary Appendix C, available at www.cmajopen.ca/content/10/4/E981/suppl/DC1.
Interpretation
In this study, we compared the detection of SARS-CoV-2 in saliva-based assays with nasopharyngeal swabs in the pediatric population. Among children who tested positive for SARS-CoV-2, the concordance rate of testing modalities was 73.3%, which is similar to other studies.2,5,6 Among our discordant results, half tested positive on saliva only, and half tested positive on nasopharyngeal swab only. This highlights the notion that there is no perfect test for the detection of SARS-CoV-2. Some children were unable to provide a saliva sample into a tube, for whom we implemented sponge-based kits, circumventing a limitation that had been reported in other studies.2
We found the probability of being asymptomatic and having discordant results to be 4 times higher than that of being symptomatic and having discordant results. Patients with discordant test results also had higher cycle threshold values overall, which suggests lower viral RNA loads.
Our findings also showed that salivary testing should be performed as close to symptom onset as possible, as viral loads decrease and cycle threshold values increase with time. This parallels results observed in adult populations using nasopharyngeal swabs, where a high viral detection rate was found when sampling 0–4 days after symptom onset, and dropped after 10–14 days.22 Further, our study highlights that frequent repeat saliva tests will increase the sensitivity of the test, making them ideal for use in repeat testing and surveillance programs.
Salivary testing for SARS-CoV-2 in the pediatric population has several advantages. Saliva testing does not require trained staff or personal protective equipment, reducing consumption of health care resources and costs during a time of high demand. Compared with nasopharyngeal swabs, saliva collection is a less invasive diagnostic test, which could improve compliance for repeat testing. Saliva testing is reliable and convenient for at-home testing, especially in remote populations with limited access to health care or testing resources.
With the presence of increasingly transmissible variants of concern, new challenges pertaining to the accessibility of accurate SARS-CoV-2 tests are introduced.23–25 Given the increased demand for PCR testing, our study shows the feasibility of saliva sampling as a convenient alternative to nasopharyngeal swab. Furthermore, both the rate of SARS-CoV-2 infections and other respiratory infections will undoubtedly increase as schools and child care centres open with fewer measures to reduce transmission.26 As such, the need for reliable and convenient serial testing in the pediatric population is required for the implementation of isolation and contact-tracing measures. Both self-collection kits used in this study are unique in that they combine saliva with a virucidal and stabilizing fluid to allow for safe and reliable transportation to a laboratory for up to 3 weeks after collection.13,14 Our study shows that it is feasible for families to bring collection kits to their home, provide a saliva sample and then ship them back to a laboratory without the help of a health care professional. This paves the way for a program of at-home self-collection, reducing children’s anxiety surrounding the testing process and minimizing the disruption to families’ daily lives in comparison to travelling to a dedicated, and possibly remote, testing centre. In addition, saliva kits can be distributed easily to families through school and child care programs in the event of local outbreaks, and their results can be used to guide return-to-school policies.
Limitations
There remains no true gold standard for SARS-CoV-2 detection in evaluation of salivary testing in adults or pediatric populations. As such, our study assumed that all positive results, on either saliva-based assays or by nasopharyngeal swabbing, are considered true positives. We did not perform a traditional sample size calculation, given the substantial variability in disease prevalence over time in the pediatric population, which is required to inform the values for the calculation. Discrepant cycle threshold values between E and RdRp genes were re-run, which may lead to discrepant analysis bias.27
Conclusion
Our study highlights the functionality, feasibility and accuracy of salivary testing for SARS-CoV-2 in a pediatric sample. Salivary testing shows similar detection of SARS-CoV-2 as testing by nasopharyngeal swab in pediatric populations, especially when children are symptomatic. Saliva testing is best performed closest to initial symptom onset, and increases in sensitivity with frequent testing, making it ideal for repetitive testing at schools.
Acknowledgements
The authors would like to acknowledge Jill Allan, Karamchand Ramotar and Johnny Ung for their contributions in making this research study possible.
Footnotes
Competing interests: Roger Zemek is a founding partner and minority shareholder of 360 Concussion Care. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Nadia Hua, Martin Corsten, Guillaume Poliquin and Stephanie Johnson-Obaseki contributed to study conception and design. Nadia Hua, Martin Corsten, Alexander Bello, Lauren Dawson, Guillaume Poliquin and Stephanie Johnson-Obaseki contributed to the acquisition of data. Nadia Hua, Martin Corsten, Alexander Bello, Rachael Milwid, David Champredon, Patricia Turgeon, Nicholas Mitsakakis, Richard Webster, Guillaume Poliquin and Stephanie Johnson-Obaseki contributed to data analysis. All authors contributed to data interpretation. All of the authors drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was funded by a Granting Contribution by the Public Health Agency of Canada.
Data sharing: These data can be made available to others upon request to the corresponding author.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/10/4/E981/suppl/DC1.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/
References
- © 2022 CMA Impact Inc. or its licensors