Abstract
Background: Many refugees and asylum seekers from countries where schistosomiasis is endemic are infected with the Schistosoma parasite when they arrive in Canada. We assessed, from a systemic perspective, which of the following management strategies by health care providers is cost-effective: testing for schistosomiasis and treating if the individual is infected, treating presumptively or waiting for symptoms to emerge.
Methods: We constructed a decision-tree model to examine the cost-effectiveness of 3 management strategies: watchful waiting, screening and treatment, and presumptive treatment. We obtained data for the model from the literature and other sources, to predict deaths and chronic complications caused by schistosomiasis, as well as costs and net monetary benefit.
Results: Presumptive treatment was cost-saving if the prevalence of schistosomiasis in the target population was greater than 2.1%. In our baseline analysis, presumptive treatment was associated with an increase of 0.156 quality-adjusted life years and a cost saving of $405 per person, compared with watchful waiting. It was also more effective and less costly than screening and treatment.
Interpretation: Among recently resettled refugees and asylum claimants in Canada, from countries where schistosomiasis is endemic, presumptive treatment was predicted to be less costly and more effective than watchful waiting or screening and treatment. Our results support a revision of the current Canadian recommendations.
Schistosomiasis is uncommon among the general population in Canada, but it frequently affects refugees and asylum claimants.1 It is a parasitic infection that causes chronic disease exclusively in people who have visited or lived in regions where the disease is endemic, such as the Middle East, Asia, the Caribbean and Africa.2,3 The disease is contracted through exposure to water contaminated with organisms known as Schistosoma spp. After a period of latent infection that may last for decades, some individuals experience complications arising from spread of schistosome eggs to various organ systems.4–10 Genitourinary disease may be associated with obstructive uropathy, glomerulonephritis, bladder cancer and female infertility.4–10 A hepatosplenic form of the disease may be associated with malabsorption, portal hypertension, pulmonary hypertension, cor pulmonale and central nervous system involvement.4–10
Refugees and asylum claimants may be asymptomatic when they arrive in their new country, but 7% to 73% have positive serologic results indicating past or current infection.11–17
Among countries that receive refugees, strategies to prevent disease vary. The Canadian Collaboration for Immigrant and Refugee Health recommends that refugees from Africa undergo screening and treatment for schistosomiasis.18 The Australasian Society for Infectious Diseases recommends screening and treatment of refugees from Africa, parts of Asia and the Middle East.19 In the United States, the Centers for Disease Control and Prevention support presumptive treatment of African refugees for schistosomiasis before their departure.20 We compared 3 strategies to determine which is cost-effective: watchful waiting, screening and treatment, or presumptive treatment. We considered presumptive treatment as an option only for refugees who have arrived in Canada, not for those who have yet to arrive, because this is an option that Canadian health care providers can easily provide if they choose to do so.
Methods
Model structure
Using Excel 2016 software (Microsoft Corporation), we developed a decision-tree model that assigned individuals to the health states shown in Figure 1, according to the probabilities of progression summarized in Table 1. The model assessed the cost-effectiveness of 3 strategies, which were as follows.
Decision tree. Note: CNS = central nervous system.
Model parameters
Watchful waiting
Under watchful waiting (status quo), we assumed that no preventive measures would be taken and that if patients became unwell they would present to hospital, where they would be treated for schistosomiasis complications. We assumed that patients with ongoing complications would receive follow-up care in the community.
Screening and treatment
Under screening and treatment, we assumed that all newly arrived refugees would be given a serologic test for schistosomiasis, which the National Reference Centre for Parasitology offers to refugee clinics across Canada. Patients with positive test results would be offered praziquantel, an antiparasitic medication that is taken over the course of a single day.
Presumptive treatment
Under the presumptive treatment strategy, we assumed that newly arrived refugees would be offered praziquantel at their first clinic visit.
Outcomes
We calculated quality-adjusted life years (QALYs) and net monetary benefit (NMB) using the formula NMB = QALYs * ($50 000/QALY) – Cost. This formula combines health and cost outcomes into a single metric, by assuming that health benefits are valued at $50 000 per QALY. We used Stata 15 software (StataCorp) to conduct linear regression of net monetary benefit against prevalence and to plot linear functions of net monetary benefit versus prevalence. Whichever option had the greatest net monetary benefit at a given disease prevalence was considered cost-effective at that prevalence.
For any 2 options that were compared, we calculated the incremental cost-effectiveness ratio (ICER) using the formula ICER = (Cost1 – Cost2)/(QALYs1 – QALYs2). However, if one option was both more effective and less costly than the other, we considered that option as “dominating” the other option, and we did not calculate the incremental cost-effectiveness ratio. Future costs and benefits were discounted at 1.5% annually, in keeping with guidelines of the Canadian Agency for Drugs and Technologies in Health.40
Model validation
We used the model and external data sources to estimate the number of cases of schistosomiasis in Calgary in a given year, and compared this estimate with case numbers in administrative data (described in detail in Appendix 2, available at www.cmajopen.ca/content/9/1/E125/suppl/DC1). We also compared the overall per-patient cost of care generated by the model and the overall disutility of symptomatic disease with estimates published in the literature (Table 2).
Disease-related costs and utilities used in the model
Model parameters and literature search
We identified studies in any language describing the natural history of schistosomiasis by searching PubMed (from inception to 2020) using the Medical Subject Heading query “Schistosomiasis/complications” and “Schistosomiasis/epidemiology” and then identifying additional studies described in reference lists (search conducted by J.W.).
We obtained probabilities of disease progression from studies conducted in countries where the disease is endemic, because studies conducted in countries where it is not endemic did not discuss specific complications.54–56 We obtained the probability of schistosomiasis affecting specific organ systems from serial autopsy studies,24,26,28 as well as the expert opinion of clinicians.27,29,30,31 Because we found no research estimating the probability of schistosomiasis-related pyelonephritis progressing to bacteremia, we used an estimate from a study of patients with indwelling urinary catheters.25
We estimated the health-related quality of life associated with various complications of schistosomiasis by obtaining utility coefficients for similar medical conditions from 3 questionnaire-based studies.41,49,53 We did not attempt to estimate the disutility of adverse effects associated with a 1-day course of praziquantel because, by virtue of being mild and lasting only a few days, they were deemed to have unimportant effects on model outcomes.
To estimate life expectancies associated with schistosomiasis complications, we used Stata 15 software to fit hazard functions to survival curves taken from disease-specific survival studies for the same conditions (Appendix 1).34–38,45 Using the hazard functions, we applied a previously published life table method to estimate mean life expectancy for people with specific complications.57 We obtained test sensitivity and specificity from data published by the National Reference Centre for Parasitology22 and a range of treatment effectiveness from field studies conducted in settings where the disease is and is not endemic.21,58 To estimate mean life expectancy for refugees, we used the Statistics Canada 2015 life table for Canadians, and applied a standardized mortality rate for refugees in Canada to the age-specific probabilities of dying.32,33
We estimated the costs of hospital-based care using costs for inpatient care published by Alberta Health,59 as well as a study on the cost of infertility treatment.52 We estimated the cost of follow-up for specific complications using published estimates and guidelines39,44,46–48,50–52,60–65 and the Alberta Health Care Insurance Plan schedules of medical and drug benefits.42,43 See Appendix 1 for further details.
The price that refugees and asylum claimants pay for praziquantel in a given province is determined by the Interim Federal Health Program, which is operated by Medavie Blue Cross on behalf of Immigration, Refugees and Citizenship Canada.66 We obtained drug costs from selected pharmacies that participate in the Interim Federal Health Program (see footnotes in Table 1). We estimated the cost of schistosomiasis testing using estimates from selected laboratories that have done full internal costing.
We derived life expectancies for healthy individuals from published research on mortality rates for refugees in Canada32 and disease-specific life expectancies from published survival rates (survival rates are described in detail in Appendix 1). We obtained age, sex and prevalence of infection from unpublished summary data for patients at the Mosaic Refugee Health Clinic in Calgary. This clinic offers schistosomiasis testing and treatment to the majority of refugees and asylum claimants in the city.
Model assumptions
We conducted our analysis from the perspective of Canadian publicly funded health care payers. We included Alberta Health, which funds labour, materials, clinic overhead, testing and treatment for residents of Alberta; Health Canada, which supports laboratory testing for schistosomiasis; and Immigration, Refugees and Citizenship Canada, which pays for asylum claimants’ health care and refugees’ prescriptions for praziquantel, under the Interim Federal Health Program.
We assumed that patients who were treated for latent infection would be given a 1-day course of praziquantel, as recommended in the current Canadian clinical guideline.18 Reported cure rates using this protocol vary between 81% and 95%.23 It is well tolerated, although some patients experience minor and transient adverse effects, such as gastrointestinal symptoms, nausea, fatigue and dizziness.58 We assumed that these would not have a substantial impact on quality of life. Because treatment lasts only 1 day, we assumed that adverse effects would not prevent individuals from finishing treatment.
Analysis of uncertainty
We calculated QALYs and net monetary benefit using both a deterministic model, with fixed costs and probabilities, and a probabilistic Monte Carlo simulation, with variable costs and probabilities. The ranges of variation for each parameter are summarized in Table 1.
We performed 1-way probabilistic sensitivity analyses that included reducing the cost of community follow-up, adherence to treatment and treatment cure rate by 50%; doubling the mean number of years to symptom onset; and assuming that patient acceptance of empiric treatment would be 15% lower than for treatment after a positive test result. We also conducted a scenario analysis in which all of these conditions were assumed to be true.
In an exploratory analysis, we regressed net monetary benefit against disease prevalence, over a range of 0% to 30%.
Ethics approval
Data collection was approved by the Conjoint Health Research Ethics Board of the University of Calgary.
Results
Model validation
As shown in Appendix 2, we compared the predicted outcomes of our model against published studies and administrative data. Our model predicted an annual cost per patient that was about half of that found in one published study56 and a utility preference for symptomatic hepatosplenic disease slightly higher than that reported in another study.67 Our model predicted a slightly higher number of symptomatic patients for the community of Calgary than was recorded in administrative data.
Base case
In our baseline analysis (Table 3), the probabilistic analysis showed that the screening and treatment approach was more effective and less costly than watchful waiting, with a cost saving of $327 and increased utility of 0.142 QALY per person. Presumptive treatment was even more effective and less costly, with an additional cost saving of almost $78 and QALY gain of 0.014 per person relative to screening and treatment. Therefore, presumptive treatment dominated both the screening and treatment option and the watchful waiting option.
Results of base case
Sensitivity analyses
One-way sensitivity analyses had no effect on the model’s results. Presumptive treatment remained dominant in every analysis: assuming 50% reductions in the cost of community follow-up, in adherence to treatment and in treatment cure rate; doubling the years to symptom onset; and assuming lower acceptance of empiric treatment. Presumptive treatment also dominated in a scenario where all of these assumptions were combined (Appendix 3, available at www.cmajopen.ca/content/9/1/E125/suppl/DC1).
Our probabilistic sensitivity analyses showed that, at the baseline prevalence, the chance that presumptive treatment would be cost-effective, relative to watchful waiting or screening, was 100% at any willingness-to-pay threshold (Appendix 4, available at www.cmajopen.ca/content/9/1/E125/suppl/DC1).
Exploratory analysis
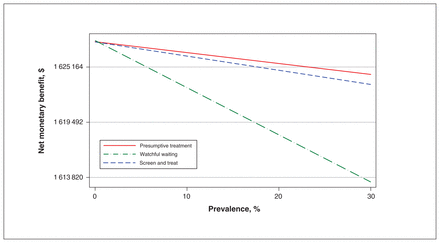
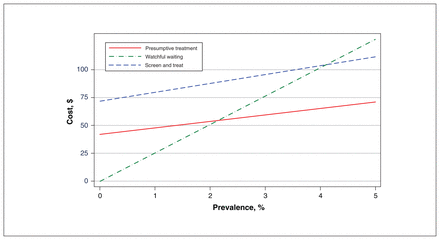
Linear regression of net monetary benefit against prevalence showed that if the prevalence is greater that 0.3%, presumptive treatment has the highest net monetary benefit and is therefore cost-effective, as illustrated in Figure 2. Figure 3 shows that when prevalence is greater than 2.1%, presumptive treatment is cost-saving by a margin that increases with prevalence and dominates the other options (for the calculations, see Appendix 5, available at www.cmajopen.ca/content/9/1/E125/suppl/DC1).
Net monetary benefit as a function of prevalence.
Cost per patient as a function of prevalence.
Interpretation
Of the 3 options that we considered in this study, presumptive treatment was the least costly and most effective. This would be true in any patient population where prevalence is greater than 2.1%. Our study estimated the prevalence at a refugee health clinic in Calgary (i.e., probability of patient being infected) to be 23.2%, whereas another study, conducted in Toronto, estimated the prevalence among HIV-positive individuals from parasite-endemic countries at 7.4%.11 Thus, we believe our results can be generalized to practices anywhere in Canada that focus on refugee health.
These results are consistent with studies that determined the cost-effectiveness of management of parasites other than schistosomes, notably that of Muennig and colleagues,68 who found that domestic presumptive treatment of immigrants to the US with albendazole, which is used to treat other parasites, would be cost-effective. When the US Centers for Disease Control and Prevention first recommended adding presumptive treatment for schistosomiasis, they assumed cost-effectiveness based on the studies for other parasites.12 After presumptive treatment for schistosomiasis began in the US, a follow-up study showed a decrease in schistosomiasis among refugees coming to California.69
The dominance of presumptive treatment is easily understood by focusing on the fundamental reasons for this finding. From the point of view of the health care system, treating an individual with praziquantel is less costly than testing that individual, whether or not treatment follows. Treating asymptomatic carriers has no serious adverse effects and prevents a small but important amount of chronic illness. That is why our cost-effectiveness acceptability curve shows presumptive treatment dominating at any level of willingness to pay (Appendix 4).
This finding raises the question of whether offering presumptive treatment to refugees before they depart their countries of origin would save additional costs, given that praziquantel may be less expensive in countries where it is available in generic form. This question may be addressed in future research.
Limitations
The model may have underestimated the cost of care for people with chronic complications of schistosomiasis. If so, this would strengthen our conclusions, because a greater amount of money would be saved by screening with treatment if necessary.
Our model predicted a slightly lower utility associated with disease than another published estimate.67 However, we believe that the difference could be explained by the published estimate having been obtained for a country with endemic disease, where study participants might have had a lower standard of living.67
Our model predicted somewhat more cases for the Calgary area than were recorded by administrative data. We believe this discrepancy can be explained by a tendency for schistosomiasis to be underdiagnosed in communities where it is rarely seen, such as Calgary.
Conclusion
Using data from a clinical practice that focused on refugees and asylum claimants, our modelling showed that presumptive treatment for schistosomiasis was more effective and less costly than the screening and treatment approach recommended in the current Canadian guideline. Our results support revision of these recommendations. However, in situations where there may be barriers to presumptive treatment, testing followed by treatment is also less costly and more effective than waiting for people who may be infected with schistosomes to become symptomatic.
Footnotes
Competing interests: Some of the aggregate data used in this study were obtained through previous research conducted by Gabriel Fabreau, supported by another research grant (without any personal fees). No other competing interests were declared.
This article has been peer reviewed.
Contributors: John Webb obtained the model parameters, designed and implemented the model, interpreted the data and drafted the manuscript. Gabriel Fabreau collaborated on study conception and provided content expertise for model development. Eldon Spackman collaborated on model development and data interpretation. Stephen Vaughan provided content expertise for the literature review and development of the model. Kerry McBrien collaborated on study conception and design and on data interpretation. All of the authors contributed to preparation and revision of the manuscript for important intellectual content, approved the final version to be published and agreed to act as guarantors of the work.
Funding: None received.
Data sharing: The data from this study are not available to others.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/9/1/E125/suppl/DC1.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/
References
- © 2021 Joule Inc. or its licensors