Abstract
Background: Cancer screening aims to detect malignant disease early in its natural history when interventions might improve patient outcomes. Such benefits are unclear when screening occurs for patients with an existing high risk of death. Our aim was to study the extent of routine cancer screening for a new primary cancer in patients with existing metastatic cancer.
Methods: We used administrative databases from Ontario to identify a retrospective cohort of adults of eligible screening age (≥ 50 yr) who had a diagnosis of stage IV (metastatic) colorectal, lung, breast or prostate cancer between 2007 and 2012. We calculated the cumulative incidence of cancer screening over time for colorectal and breast cancer.
Results: Among the 20 992 patients with metastatic lung, breast or prostate cancer, 2.9%, 6.3% and 13.3% of patients, respectively, underwent testing for colorectal cancer within 1 year of cancer diagnosis. Within 3 years of diagnosis, rates reached 4.1%, 12.3% and 27.5%, respectively (8.5% of all patients). Incidence of colorectal cancer testing was higher among patients who received their diagnoses more recently compared with patients with diagnoses from earlier time periods (p = 0.0143). Among the 10 034 women with metastatic lung or colorectal cancer, 8.7% and 8.0% of patients, respectively, underwent breast cancer screening within 1 year of cancer diagnosis. Within 3 years of diagnosis, screening rates reached 10.2% and 13.1%, respectively.
Interpretation: Our findings indicate excessive rates of cancer screening among patients with metastatic cancer who are unlikely to benefit. Further studies are warranted to identify predictors for screening, resource implications, potential and real harms borne by patients, and the impact of a recent Choosing Wisely statement recommending against the practice.
As the prevalence of cancer increases and the cost of care rises in parallel, it becomes increasingly important for resources for cancer care to be judiciously applied. Screening for breast and colon cancer is now common owing to their demonstrated benefit when early stage malignant disease can be detected.1–6 However, for patients who already have metastatic cancer, the long-term benefit of screening for a new primary cancer is unlikely to be realized and is associated with potential harm and unnecessary resource consumption. Although the prognosis associated with metastatic disease varies (with 5-year survival ranging from about 5% for lung cancer to 30% for prostate cancer),7,8 most patients with stage IV (metastatic) disease at presentation would not be expected to derive long-term benefit from undergoing screening for asymptomatic early stage malignant disease. Furthermore, excessive screening could impose additional medical complications and discomfort (i.e., related to the screening procedures themselves or to subsequent investigations), added costs and psychological burden on patients at their most vulnerable. These risks are unlikely to be offset by any improvement in life expectancy or quality of life for this unique patient population.
Choosing Wisely Canada aims to initiate conversations about unnecessary treatments and procedures and guide high-quality care.9 In particular for cancer, the campaign seeks to reduce unnecessary interventions that are not supported by evidence or could contribute inordinately to the rising cost of cancer care (www.choosingwiselycanada.org/recommendations/oncology/). In 2013, a task force that included representatives from the Canadian Association of Radiation Oncology, Canadian Association of Medical Oncology and Canadian Society for Surgical Oncology was convened to develop cancer-specific Choosing Wisely Canada statements. The initial list included the statement “Don’t perform routine cancer screening, or surveillance for a new primary cancer, in the majority of patients with metastatic disease.” However, when this statement was issued, it was unclear whether the practice of inappropriate screening occurred in Canada, in particular within a setting in which population-based screening programs for breast and colorectal cancer are prevalent and promoted. We sought to evaluate the performance of routine cancer screening for a new primary cancer among patients with existing metastatic cancer and understand the trend of this practice over time. We hypothesized that a small proportion of patients with metastatic disease were undergoing screening for a new unrelated primary cancer, despite being unlikely to benefit from the practice.
Methods
Sources of data
We used population-based administrative health care databases from Ontario that are held at the Institute for Clinical Evaluative Sciences (ICES). The following data sets were linked using unique encoded identifiers and analyzed at ICES: the Ontario Cancer Registry,10 the Registered Persons database, the Ontario Health Insurance Plan (OHIP),11 the Canadian Institute for Health Information (CIHI) Discharge Abstract Database, the Ontario Breast Screening Program (OBSP),12 and the Ontario Crohn’s and Colitis database.13
Design
Our retrospective cohort included all adult residents of Ontario of eligible screening age who had received a diagnosis of incident colorectal, lung, breast or prostate cancer between Jan. 1, 2007, and Dec. 31, 2012 (with follow-up to Dec. 31, 2013). This period represented the most recent period for which comprehensive data were available within all of the administrative databases used for linkage. Only patients who had stage IV (metastatic or noncurable) cancer at diagnosis were included, owing to the availability of an administrative database code for metastatic disease available within the Ontario Cancer registry. We further restricted the cohort to patients aged 50 years and older at the time of diagnosis, based on the recommended age for breast and colorectal screening in Canada.12,14 We excluded patients who had a previous history of any of the 4 cancers of interest, patients for whom multiple cancers were diagnosed on the same date, and patients for whom stage information was not available at the time of diagnosis.
Outcomes
Outcomes of interest were the occurrence of breast cancer screening using mammography and use of colorectal tests as a marker of colorectal cancer screening.
Colorectal cancer tests included fecal occult blood testing, flexible sigmoidoscopy or colonoscopy. In Ontario, a population-based province-wide screening program for colorectal cancer (ColonCancerCheck) recommends that people aged 50 to 74 years undergo fecal occult blood testing every 2 years (or flexible sigmoidoscopy every 10 years). For people with an increased risk of colorectal cancer (i.e., family history in 1 or more first-degree relatives), colonoscopy at 50 years of age, or 10 years earlier than the age at which their relative’s diagnosis was made, is recommended (www.cancercare.on.ca/pcs/screening/coloscreening/cccworks/).14
Breast cancer screening was defined by mammography through the Ontario Breast Screening Program (OBSP) or an OHIP record of bilateral mammography. The OBSP (www.cancercare.on.ca/pcs/screening/breastscreening/OBSP/) is a province-wide breast screening program that provides high-quality screening investigations for women of average risk and aged 50 to 74 years (in addition to women with increased risk who are aged 30–69 yr).12
We documented colorectal cancer screening among patients with lung, breast and prostate cancer, and excluded patients with a history of colorectal cancer, including those for whom it was their incident cancer diagnosis, and those with a previous history of inflammatory bowel disease. For the breast screening analysis, we documented screening among women with lung cancer and colorectal cancer, and excluded male patients and women with breast cancer as their incident cancer diagnosis. We estimated screening rates stratified by age: 50–74 years, and 75 years and older. Given the high mortality of this population, the incidence of screening was calculated using the cumulative incidence function, which takes into account the competing risk of death or the occurrence of the cancer for which the patient was undergoing screening (before screening).
Statistical analysis
We estimated the cumulative incidence of cancer screening within 1 and within 3 years after diagnosis; only the first screening tests after the cancer diagnosis were counted. We further investigated whether the year of diagnosis (from 2007 to 2012) affected the probability of subsequent screening (to assess whether the probability of screening changed over time). A Fine–Gray subdistribution hazards model was used to study the effect of year of diagnosis on the incidence of cancer screening. 15 Given that the indication for colonoscopy is not available in health administrative data, we performed a sensitivity analysis for a subcohort of patients with breast, lung or prostate cancer who were deemed eligible for colorectal screening because they had not undergone FOBT in the previous 2 years, sigomoidoscopy in the previous 5 years and colonoscopy in the previous 10 years before their cancer diagnosis.
Ethics approval
This study was approved by the institutional review board at Sunnybrook Health Sciences Centre.
Results
Colorectal cancer screening
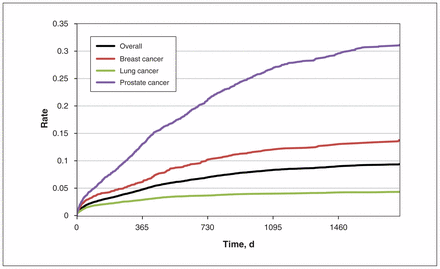
We identified 20 992 patients with an incident diagnosis of metastatic lung, breast or prostate cancer for our analysis (Table 1). Colorectal cancer tests within 1 year of receiving an advanced cancer diagnosis occurred in 2.9%, 6.3%, and 13.3% of patients with lung, breast and prostate cancer, respectively (4.9% across the 3 diagnoses combined). The probability of undergoing screening was higher for the subgroup of patients aged 50–74 years (compared with patients older than 75 yr). Fecal occult blood testing and colonoscopy were used with similar frequency (Table 1). Within 3 years of diagnosis, screening rates reached 4.1% for patients with lung cancer, 12.3% for patients with breast cancer, and 27.5% for patients with prostate cancer (8.5% across all patients combined; Figure 1). In a sensitivity analysis involving the subcohort of patients who were deemed screen-eligible (n = 12 056), the 1-year screening rate across all cancers was 3.7%, and the 3-year screening rate was 6.0%.
Screening rates among patients with stage IV (metastatic) colorectal, lung, breast or prostate cancer
Testing for colorectal cancer among patients with stage IV (metastatic) lung, breast or prostate cancer.
Breast cancer screening
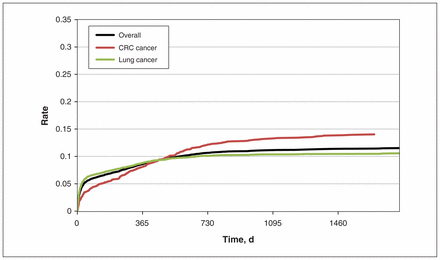
We identified 10 034 women with metastatic lung or colorectal cancer (Table 1). Breast cancer screening within 1 year after receiving an advanced cancer diagnosis occurred in 8.7% of women with lung cancer and 8.0% of women with colorectal cancer (8.5% across all patients combined). A higher probability of screening was again seen among patients aged 50–74 years. Within 3 years of diagnosis, screening rates reached 10.2% among patients with lung cancer and 13.1% among patients with colorectal cancer (11.0% of all patients; Figure 2).
Breast cancer screening in patients with stage IV (metastatic) lung or colorectal cancer (CRC).
Screening rates over time
Screening rates were further analyzed by calendar year (Table 2). Screening for colorectal cancer increased from a cumulative incidence of 4.11% (95% confidence interval [CI] 3.45%–4.84%) in 2007 to 5.04% (95% CI 4.12%–6.09%) in 2012. Based on the Fine–Gray regression model we used to study the effect of year of diagnosis on incidence of screening, the incidence of colorectal cancer screening increased over the study period (hazard ratio [HR] 1.05; 95% confidence limits 1.01–1.09; p = 0.01]). In contrast, the incidence of breast cancer screening remained stable over the same period (HR 0.97; 95% CI 0.93–1.01; p = 0.2).
Screening by calendar year, 2007–2012
Interpretation
Our findings suggest that a substantial proportion of patients with metastatic cancer with a prognosis known to be poor underwent screening for new primary cancers. Screening in this population is not only an inappropriate use of scarce health care resources, but could detract from the quality of care required by patients near the end of life. Such screening exposes patients to potential harms in addition to extra testing, time, stress and financial burden, but without a likelihood of benefit from a patient perspective.
Despite these concerns, nearly 1 in 20 patients in our cohort underwent tests for colorectal cancer and nearly 1 in 11 women in our cohort underwent screening for breast cancer within a year of receiving their diagnosis of metastatic disease. Not surprisingly, screening rates for both colorectal and breast cancer were higher among patients aged 50–74 years, which includes the recommended ages in Ontario for screening for patients at average risk. There is a potential that this excessive screening reflects and parallels the successful promotion of breast and colorectal cancer screening activities among Ontario physicians,12,14 albeit without the careful individualized considerations that should encompass all patient care. Screening programs such as the OBSP clearly provide benefit at a population level, but physicians should be actively involved in assessing individual patients and engaging them in a discussion regarding their specific risks and benefits related to screening evaluations. In particular, in the context of metastatic cancer, engaging patients in a conversation around underlying prognosis and potential screening outcomes can be challenging; however, physicians should be guided by the overarching principles of patient-centred care in their endeavour to better understand patient preferences.16
We noted some variability in the screening rates according to the underlying metastatic diagnosis. Screening rates for both colorectal and breast cancer were lowest among patients with advanced lung cancer, potentially reflecting the anticipated short survival associated with this stage of the disease (4.5% survival at 5 years for patients with metastatic disease).7 In contrast, very high rates of colorectal cancer testing were seen among men with prostate cancer, reaching 13% at 1 year and 27% at 3 years. Again, this may relate to a perception that metastatic prostate cancer may not be imminently fatal (29.8% survival at 5 years for patients with metastatic disease). 8 We were unable, in this study using administrative databases, to determine whether screening was preferentially offered to patients who were perceived to have improved survival. For patients with metastatic prostate cancer, an alternative explanation for increased screening may be that a proportion of the colonoscopies were completed for rectal bleeding (i.e., related to radiation therapy) and not for screening purposes. However, it is notable that fecal occult blood testing was the most common screening modality for colorectal cancer, and even it was excessively applied in our study cohort. This may have led to increased subsequent investigations in addition to unmeasured patient fear and anxiety owing to abnormal test results.
Our work builds upon a previous study of screening among older adult Medicare enrollees with advanced cancer, reported within the Surveillance, Epidemiology, and End Results (SEER) tumour registries in the United States.17 In this earlier study, 8.9% of women received at least one screening mammogram following a diagnosis of advanced malignant disease, and 1.7% of all patients with advanced cancers received lower gastrointestinal endoscopy. Accounting for differences between our studies related to cohort selection and the window for event determination, the rates of breast cancer screening appear to be commensurate, whereas the rates of lower gastrointestinal endoscopy appear to be higher in our study. Some of these differences may relate to the younger population included in our study, a group for which more aggressive care might be offered. Moreover, we note that the SEER study focused on an earlier time frame (1998–2005) than ours (2007–2012). Our time trend analysis did show a continued increase in the use of colonoscopy, but a relatively stable use of screening mammograms, which could explain the differential findings. Our current study was unable to determine the underlying factors that might drive the increase in colorectal cancer screening over time; these factors warrant further investigation.
Limitations
We did not include a control population of patients without advanced malignant disease for comparative baseline screening rates because our premise was that any screening that occurred among patients with metastatic cancer is likely inappropriate. We narrowed our population to include patients with metastatic disease at diagnosis, thereby excluding patients with earlier stage disease and subsequent progression; however, this population likely represents the cohort least likely to benefit from screening. As noted above, colonoscopy indication was not available for most of the colonoscopies performed in our cohort; therefore, we cannot rule out that a proportion were done to investigate symptoms such as bleeding and not for screening purposes. Thus, the practice of screening based solely on our estimates of colonoscopies would potentially be overestimated. However, the rate of fecal occult blood testing remained high in all groups, suggesting that even with this modality alone, screening was excessive. In addition, our data do not allow us to determine the reason for bilateral mammograms identified by OHIP (for example, some may have been performed for symptomatic masses detected on physical examination). Finally, the databases we used are specific to Ontario; we are unable to determine whether our findings are broadly generalizable across Canada.
Conclusion
Our findings suggest inappropriate use of screening among patients with metastatic cancers. Further investigation should be done to identify the reasons for this practice, in addition to the costs to the health system, the financial costs borne by patients (including lost productivity and additional indirect costs), and the nonfinancial implications for patients and caregivers (including harms resulting from screening and impact on quality of life and anxiety). Our study suggests that education of physicians and the general population is needed regarding the lack of utility of cancer screening for patients with noncurable cancer in addition to further study into its effects on patients; the Choosing Wisely Canada campaign represents an important starting point in highlighting such interventions that are unlikely to offer benefit to patients.
Acknowledgements
Dr. Cheung is supported by funding from Marjorie and Roy Linden and Joan Fisher and James Rowland. Dr. Austin is supported in part by a Career Investigator Award from the Heart and Stroke Foundation of Canada (Ontario Office). Dr. Tinmouth was supported in part by a University of Toronto Department of Medicine Clinician Scientist Award at the time of this study.
Footnotes
Competing interests: Jill Tinmouth was the lead scientist for the ColonCancerCheck program at Cancer Care Ontario. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Matthew Cheung, Hadas Fischer and Simron Singh contributed to the conception and design of the study, collection and assembly of data, data analysis and interpretation and manuscript writing. Jill Tinmouth contributed to the data analysis and interpretation, and manuscript writing. Kinwah Fung and Peter Austin contributed to the data collection and assembly, data analysis and interpretation, and manuscript writing. All of the authors approved the final version of the manuscript and agreed to act as guarantors of the results.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/6/4/E538/suppl/DC1.
Disclaimer: This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred.
Parts of this material are based on data and/or information compiled and provided by Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions and statements expressed in the material are those of the authors, and not necessarily those of CIHI. Parts of this material are based on data and information provided by Cancer Care Ontario (CCO). The opinions, results, view, and conclusions reported in this paper are those of the authors and do not necessarily reflect those of CCO. No endorsement by CCO is intended or should be inferred.
References
- Copyright 2018, Joule Inc. or its licensors