Abstract
Background Childhood obesity is a public health concern. One-third of North American children and youth are overweight or obese. We reviewed the evidence of behavioural and pharmacological weight-management interventions on body mass index (BMI), BMI z-score and the prevalence of overweight and obesity in children and youth.
Methods We updated the search of a previous review. We searched 4 databases up to August 2013. We included randomized trials of primary care–relevant behavioural (diet, exercise, lifestyle) and pharmacological (orlistat) interventions for treating overweight and obesity in children and youth aged 2–18 years if 6-month post-baseline data were provided for BMI, BMI z-score or prevalence of overweight and obesity. In addition, we examined secondary health outcomes such as lipid and glucose levels, blood pressure, quality of life and physical fitness. We included any study reporting harms. We performed meta-analyses when possible, and we examined the features of interventions that showed benefits.
Results Thirty-one studies (29 behavioural, 2 pharmacological and behavioural) were included. Both intervention types showed a significant effect on BMI or BMI z-score in favour of treatment (behavioural: standardized mean difference [SMD] –0.54, 95% confidence interval [CI] –0.73 to –0.36; orlistat plus behavioural: SMD –0.43, 95% CI –0.60 to –0.25). Studies reported no significant difference between groups in the likelihood of reduced prevalence of overweight or overweight and obesity. Pooled estimates for blood pressure and quality of life showed significant benefits in favour of treatment (systolic blood pressure mean difference [MD] –3.42, 95% CI –6.65 to –0.29; diastolic blood pressure MD –3.39, 95% CI –5.17 to –1.60; quality of life MD 2.10, 95% CI 0.60 to 3.60). Gastrointestinal difficulties were more common in youth taking orlistat than in the control group (risk ratio 3.77, 95% CI 2.56 to 5.55). We saw much variability across efficacious interventions.
Interpretation Low- to moderate-quality evidence suggests behavioural treatments are associated with a medium effect in terms of reduced BMI or BMI z-score compared with a small effect shown by combined pharmacological–behavioural interventions. Future research should evaluate active weight maintenance interventions in adolescents with longer follow-up and examine the effectiveness of combined pharmacological and behavioural interventions. Registration: PROSPERO no. CRD42012002754
According to the World Health Organization, school-aged children and youth (aged 5–19 years) whose weight is greater than the 85th centile are overweight, and those whose weight is above the 97th centile are obese; younger children (aged 2–5 years) must be over the 97th centile to be considered overweight and more than the 99.9th centile to be considered obese.1 A recent Canadian Health Measures Survey (2009–2011) reported obesity prevalence among 5- to 17-year-olds at 11.7%, with an additional 19.8% classified as overweight.2 In the United States, obesity prevalence among 2- to 19-year-olds (2009–2010) was reported at 16.9%, with another 14.9% considered overweight.3 Obesity that begins in childhood usually persists into adulthood4 and is associated with adverse outcomes including metabolic, cardiovascular, musculoskeletal, neurologic, gastrointestinal, respiratory and psychosocial disturbances.5–10 The predicted increase in childhood obesity has intensified the urgency of improving treatment approaches for the pediatric population.
Treatment of childhood and adolescent obesity is an active area of research, and a number of systematic reviews have been published recently.11–17 Comprehensive behavioural interventions including changes in diet, physical activity and lifestyles involving individual patients or families are commonly used and generally considered primary modes of treatment.18–21 Recent research has focused on establishing the efficacy of combining pharmacological agents such as orlistat with conventional behavioural interventions, especially in adolescents with severe obesity, but these drugs are associated with potential adverse events.22–24 We aimed to provide an updated synthesis of the evidence on benefits and harms of overweight and obesity treatment interventions for children and adolescents feasible for use in or referral from primary care, and we examined the features of efficacious interventions.
Methods
Search strategy
A recent high-quality review (9/11 AMSTAR25 rating) by the US Preventive Services Task Force examined the effectiveness of weight management programs for children with overweight and obesity.17 We evaluated their search and determined that it addressed our key questions. To avoid duplication, we planned to bring forward any of their included studies that met our criteria. To update the evidence we used the same search strategy with 3 modifications: we did not use the Database of Abstracts of Reviews of Effects (DARE) or Education Resources Information Center (ERIC) database, but we added Embase; we changed the dates covered in the search; and we added a filter to limit studies to those published in English or French given our limited resources for handling papers in multiple languages. We searched Embase, MEDLINE, the Cochrane Central Register of Controlled Trials and PsycINFO from Jun. 10, 2008 (the date of the last US Preventive Services Task Force search) to Aug. 28, 2013 (our search strategy is outlined in Appendix 1, available at www.cmajopen.ca/content/3/1/E35/suppl/DC1). In addition, we searched the reference lists of included studies and pertinent reviews for additional relevant studies not captured by our search.
Inclusion and exclusion criteria
Details regarding the population, interventions, comparators, outcomes and settings (PICOS) criteria for this review are provided in Box 1, and the inclusion and exclusion criteria are shown in Box 2.
Study selection, quality assessment and data abstraction
Titles and abstracts were reviewed in duplicate. Citations marked for inclusion by either team member underwent full-text screening, which was also done independently by 2 people. One person completed full abstraction, and a second person verified extractions. Data were checked again before analysis. We assessed randomized controlled trials using the Cochrane risk-of-bias tool.27 We determined the overall strength of the evidence using the Grading of Recommendations Assessment, Development and Evaluation system (GRADEpro version 3.2); we applied ratings of high, moderate, low or very low based on assessments of 5 domains of the evidence (risk of bias, indirectness, imprecision, inconsistency and reporting bias). We resolved all conflicts through discussion between raters and, if necessary, through consultation with review team members.
Data analysis
For meta-analyses, we used means and standard deviations (SD) for continuous outcomes (e.g., BMI) and counts data for binary outcomes (e.g., prevalence, adverse events). Whenever possible, we used immediate posttreatment data; otherwise, we selected the data point closest to the end of the intervention and at least 6 months after baseline. If studies reported results for boys and for girls, we entered these data separately. For studies with multiple intervention arms, we combined data from similar groups (e.g., 2 lifestyle arms, 1 delivered to families, 1 delivered only to parents) to do a pairwise comparison with the control group.27 We used Cochran Q (α = 0.05) and I2 (≥ 75% = high heterogeneity) statistics to quantify statistical heterogeneity between studies. We used RevMan version 5.3, STATA version 12 and GRADEpro for statistical analyses.
We chose standardized mean difference (SMD) as a summary measure of effect to allow us to combine data for BMI and BMI z-score in a single meta-analysis; if a study reported both outcomes, we used the nonstandardized data. This strategy, which is consistent with the approach taken in other reviews17,28 increases the pool of studies, thereby increasing the power to detect a difference in weight change between groups. We used the DerSimonian and Laird random effects model with inverse variance29 to generate SMDs for BMI and BMI z-score (< 0.2 = very small effect; ≥ 0.2 to < 0.5 = small effect; ≥ 0.5 to < 0.8 = medium effect; ≥ 0.8 = large effect).30 We used this same random effects model20 to generate summary measures of effect in the form of mean differences (MDs) for the other continuous outcomes and risk ratios (RRs) for binary outcomes. For harms, we calculated absolute risk increase (ARI) and number-needed-to-harm (NNH); the latter were calculated using the absolute numbers computed by the GRADE software. GRADE estimates the absolute number per million using the control group event rate and RR with the 95% confidence interval (CI) obtained from the meta-analysis. For BMI and BMI z-score, we conducted subgroup analyses based on the focus of intervention — behavioural or pharmacological (orlistat) plus behavioural — and then only for behavioural approaches based on intervention type (diet, exercise, diet plus exercise, lifestyle), intervention duration (≤ 12 mo, > 12 mo), age group (2–12 yr, 13–18 yr), intervention target (individual, family) and study risk of bias rating (low, unclear, high).
Efficacious interventions were identified from studies in the BMI and BMI z-score meta-analysis that significantly favoured treatment. Our choice of intervention characteristics to examine (target sex and age, estimated number or frequency of sessions, group sessions, family involvement and staff training) was informed by a similar list in a previous systematic review,17 to which we added intervention duration, type and setting, based on our belief that primary care professionals might want to consider these additional features when making referrals or recommending programs to patients and their families.
Results
Search and selection
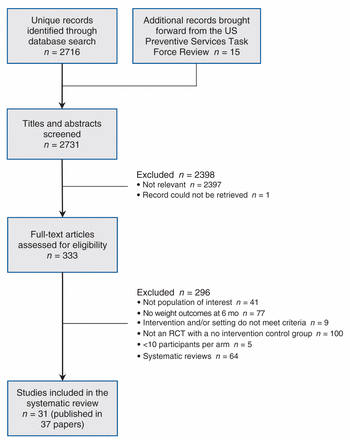
We conducted title and abstract screening on 2716 citations located through our updated search (Figure 1). We added 15 older studies that appeared in the US Preventive Services Task Force review17 to the pool of recently published papers retained for full-text screening (n = 319). We included 31 studies (published in 37 papers) in our analysis, 9 of which were brought forward from the US Preventive Services Task Force review,31–39 and 22 of which were identified from the more recent literature.40–61
Selection of studies for inclusion in the systematic review and meta-analyses.
Characteristics of included studies
Characteristics of the included studies are summarized in Table 1. All but 1 study41 included both male and female participants. Most (n = 23) studies involved elementary school–aged children (mean age at baseline 5–12 yr), 19 studies involved children and youth with overweight or obesity, and 11 targeted children and youth with obesity only. Multicomponent behavioural interventions (lifestyle or diet plus exercise) were used in 26 studies, and a combined pharmacological (orlistat) and behavioural approach was used in 2 studies. Interventions targeted families in 18 studies and individuals in 13 studies. Almost all (n = 28) of the interventions lasted one year or less, and more than half (n = 18) lasted between 2 and 6 months. Additional details of the individual studies are provided in Appendix 2 (available at www.cmajopen.ca/content/3/1/E35/suppl/DC1).
Twenty-eight of the studies were rated as having unclear or high risk of bias for the weight outcomes (Table 2), primarily due to the lack of information about or lack of procedures to ensure random sequence generation, allocation concealment and blinding of participants, personnel and outcome assessment.
Change in BMI and BMI z-score
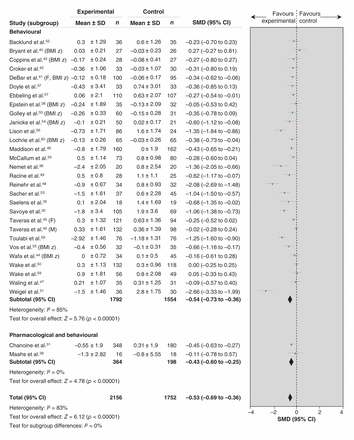
Thirty studies were included in the meta-analysis assessing change in BMI and BMI z-score.31–60 We found a significantly lowered BMI and BMI z-score in the intervention group compared with the control group with a medium size of effect (Figure 2 and Table 3). The subgroup analysis based on intervention focus (i.e., behavioural alone v. pharmacological plus behavioural) showed no difference in reduction of BMI and BMI z-score (Table 3). Further sensitivity analyses for behavioural intervention studies showed no difference in reduction of BMI and BMI z-score across treatment types, intervention duration, participants’ age or risk-of-bias rating (Table 3). There was, however, a difference in reduction in BMI and BMI z-score depending on the target of intervention; treatments focusing on the individual children (e.g., classroom interventions with no parent involvement) had a large effect, whereas family-based approaches (e.g., active parent involvement) showed a smaller, but still significant, effect (Table 3). The study that could not be pooled found no significant (p = 0.86) treatment effect on BMI z-score for a 6-month lifestyle intervention targeted at children aged 5–8 years.61
Effect of treatment interventions on body mass index and body mass index z-score (behavioural, pharmacological plus behavioural). Note: BMI z = body mass index z-score, F = female, M = male, SMD = standardized mean difference.
Four trials reported follow-up data (6–12 mo postintervention).32,41,57,60 Meta-analysis showed significantly lower BMI and BMI z-score in the intervention group compared with the control group by the end of treatment (Table 3). However, there was no difference in BMI and BMI z-score between groups at the time of postintervention to 1-year follow-up.
Change in BMI
We included 21 trials that assessed change in BMI in our meta-analysis.31,32,34–37,39,42,45–53,56–59 Intervention participants had a significantly greater reduction in BMI compared with participants in the control groups (Table 4). Our subgroup analysis based on intervention focus (i.e., behavioural alone v. pharmacological plus behavioural) showed no significant difference in reduction of BMI.
Change in prevalence of overweight/obesity
Three low-quality trials (downgraded for risk of bias and imprecision) provided results for change in prevalence of overweight or obesity that could not be pooled because they used different weight categories (overweight, overweight/obesity, obesity), and 1 study did not provide events data.35,36,50 The 2 studies that included elementary school–aged children reported prevalence 9 months after 3-month family-based interventions involving diet and exercise. No difference between groups was seen in 1 study (n = 242) (RR 0.93, 95% CI 0.82 to 1.06);50 in the second study (n = 40), there was a 5%–6% reduction in obesity prevalence in the intervention group.36 The third study reported no significant difference in change in prevalence between intervention and control groups 3 months after a 4-month lifestyle program for youth with overweight or obesity youth (n = 38) (RR 0.90, 95% CI 0.54 to 1.46).35
Change in other health outcomes
We examined changes from baseline to postintervention in cardiometabolic outcomes associated with treatment. Blood pressure outcomes were reported in 5 studies,31,48,51,53,55 and significant changes in systolic (MD –3.42, 95% CI –6.65 to –0.29) and diastolic (MD –3.39, 95% CI –5.17 to –1.60) blood pressure were seen (Table 4). No significant differences in any lipid variables (total cholesterol, low- or high-density lipoproteins or triglycerides) were seen (Table 4).31,32,39,41,49,55 One study provided data on fasting glucose levels, and no significant difference was seen (Table 4).31 None of the included studies reported changes in physical fitness as measured by laps or stages of the multistage fitness test.26
Six trials examined changes in quality of life after treatment for obesity.34,41,42,44,50,55 Five studies used the 23-item Pediatric Quality of Life inventory (PedsQL),62 and 1 study used the 37-item DISABKIDS Chronic Generic Measure.63 Both tools are validated instruments for use with children and adolescents, and access domains of physical, emotional, social and psychosocial functioning. Meta-analysis showed a significant improvement in overall quality-of-life scores in the intervention groups compared with the control groups (SMD 2.10, 95% CI 0.60 to 3.60, Table 4).
Harms
Nine studies provided data for adverse effects of treatment.31,39,42,46,49,50,57–59 Meta-analyses showed no significant differences between intervention and control groups for the categories of any adverse events, serious adverse events (requiring admission to hospital or urgent medical care) and study withdrawal due to adverse events (Table 5). One study31 reported that gastrointestinal disturbances (e.g., bloating and diarrhea) were significantly more common with orlistat treatment compared with the control (RR 3.77, 95% CI 2.56 to 5.55; number needed to harm 3, 95% CI 2 to 5) (Table 5).
Features of efficacious interventions
Sixteen studies showed a significant benefit for participants in treatment arms compared with control arms in terms of reduced BMI and BMI z-score (Figure 2). We designated the interventions in these studies as efficacious (Table 6 and Appendix 3, available at www.cmajopen.ca/content/3/1/E35/suppl/DC1).
The focus of the behavioural interventions varied and included diet (n = 2), exercise (n = 1), diet and exercise combined (n = 4) and lifestyle (n = 8). Eleven interventions involved group sessions, 5 used individual sessions, and almost all interventions (n = 12) incorporated parental or family involvement.32,35,36,41,48,49,51,53–55,58,60 Three interventions used technology to facilitate interaction between participants and study personnel or as a means of delivering information or encouraging physical activity. Duration of interventions ranged from 3 months to 2 years. All but 1 intervention lasted 1 year or less, and most (n = 11) were in place for 6 months or less. The number and frequency of sessions varied; however, most strategies involved weekly or biweekly contact with participants; a few interventions were more intense, interacting with participants twice or more each week. Most interventions were offered to male and female participants (n = 14) and two-thirds (n = 10) targeted elementary school–aged children. About half (n = 7) of the interventions were done in the United States, and one-third (n = 5) took place in European countries.
The efficacious intervention that used a pharmacological plus behavioural strategy targeted male and female adolescents with obesity in Canada and the United States. The treatment combined a 120 mg dose of orlistat taken 3 times daily with a standard dietary intervention and encouragement to engage in regular physical activity. After a 2-week lead-in period, the intervention ran for 1 year.
Interpretation
The meta-analyses of BMI and BMI z-scores in this updated systematic review of treatments for overweight and obesity in children and youth showed moderate benefits for treatment compared with control, and these findings are consistent with previous reviews.14,15,17,64 We used a comprehensive approach of subgrouping studies for behavioural interventions to reflect the evidence in existing literature. In addition to conventional measures of obesity, such as BMI and BMI z-score, we looked at prevalence of overweight and obesity to help quantify the clinical significance of weight loss. Although limited in the number of studies, our review of cardiometabolic outcomes suggests modest declines in BMI are accompanied by declines in blood pressure, which is consistent with other reviews.14,64 We also identified improvement in quality-of-life scores. In contrast to weight loss of 5%–10% in adults,65 a threshold associated with improved health outcomes for children has not been established. In addition, Kolotourou and colleagues argue BMI is too restrictive an outcome, and that additional outcomes such as fitness, self-esteem, physical and sedentary activities should be measured.66
Four studies evaluated the sustainability of changes in BMI after completion of weight-management programs. Unfortunately, after 6 months, no impact of the interventions on BMI was identified. Although this result is consistent with known biological adaptations to weight loss, it highlights the challenge of introducing time-delimited weight management interventions without follow-up, the need to introduce innovative approaches to pediatric weight management and the need to identify ways of maintaining interventions over the long-term. Studies are not yet available in the pediatric population, but it is apparent that sustained weight loss is possible in adults when interventions are maintained at a lower intensity.67
Limitations
Most of the evidence was taken from studies assessed as having an unclear risk of bias, and potential reporting bias was identified across a number of outcome- and comparison-based study groupings. In our main outcome of BMI and BMI z-score, statistical heterogeneity was high. In addition, the results presented for other health outcomes should be interpreted with caution, because we only included studies that also reported our weight outcomes. Finally, including papers published only in English or French meant possible data for relevant interventions available only in other languages were not captured.
Conclusion
Behavioural interventions for treating overweight and obesity in children and youth are associated with a moderate treatment effect in terms of a lowered BMI and BMI z-score. A small treatment effect is seen in combined pharmacological and behavioural interventions. The benefits of behavioural approaches are achieved with minimal or no adverse effects, and low-intensity behavioural interventions could readily be implemented in certain primary care settings. Few studies followed participants after completion of the intervention, but those that did found differences in BMI between groups were not maintained. Given that few studies specifically targeted youth or lasted more than 12 months, future research should evaluate active maintenance interventions in adolescents with longer follow-up. Furthermore, limited evidence as to the effectiveness of combined pharmacological and behavioural interventions warrants future research in this context.
Supplemental information
For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/3/1/E35/suppl/DC1
PICOS (population, interventions, comparators, outcomes and settings) criteria
Population
• Children and youth aged 2–18 yr with overweight or obesity (body mass index [BMI] > 85th percentile for age and sex)
Interventions
• Behavioural (diet, exercise, lifestyle), pharmacological (orlistat) or combined treatments for weight loss or management
Comparators
• Treatment effectiveness — no intervention, usual care, placebo or minimal intervention (e.g., newsletter or single information session on healthy living)
• Treatment harms — any type of comparison group or no comparison group
Outcomes
• Treatment effectiveness — primary weight outcomes: changes in BMI, BMI z-score and prevalence of overweight or obesity; secondary health outcomes: changes in total cholesterol, high- and low-density lipoproteins, triglycerides, fasting blood glucose, systolic and diastolic blood pressure, overall quality of life and physical fitness (measured using the multistage 20-metre shuttle run test)26
• Treatment harms — any adverse events, serious adverse events (requiring admission to hospital or urgent medical care), gastrointestinal events, withdrawal from study due to adverse events
Settings
• Generalizable to Canadian primary care or feasible for use in or referral from primary care; interventions should be initiated through (or feasible within) a primary care setting and (could be) delivered by a health care professional (e.g., physician, psychologist, nurse, dietician)
• Surgical and metabolic unit interventions were excluded as representing a level of obesity and comorbid conditions that would be less commonly used as referral point from primary care
Inclusion and exclusion criteria
Studies were included if they met the following criteria:
• Trials of behavioural (diet, exercise, lifestyle strategies), pharmacological (orlistat) or combined (behavioural and pharmacological) weight loss treatment or management
• Intervention targeted children and youth aged 2–18 yr with overweight or obesity (body mass index [BMI] > 85th percentile for age and sex)
• Randomized controlled trial with a no intervention, usual care, placebo or minimal component (e.g., single newsletter or information session on general health) comparison group
• Reported data for one or more specified weight outcomes (change in BMI, BMI z-score or prevalence of overweight or obesity)
• Reported data for outcomes of interest at least 6 months after baseline assessment
• Enrolled at least 10 participants in each group
• If a study reported data for harms of treatment, they were included regardless of the above restrictions on study design, comparison group, weight outcome reporting, timing of assessment or sample size
• Results were published in English or French
Studies were excluded if:
• Treatment involved changes in the built environment (e.g., neighbourhood design, transportation options, access to playgrounds or green space), surgery or drugs other than orlistat (i.e., drugs not approved for weight loss by Health Canada)
• The study specifically enrolled participants who had an eating disorder or a condition in which weight gain was a cardinal manifestation (e.g., Prader–Willi syndrome, polycystic ovarian syndrome, pregnancy)
• Intervention was conducted in an inpatient hospital setting or involved a faith-based program
• Published results were only available in a language other than English or French
Acknowledgements
Parminder Raina holds a Tier 1 Canada Research Chair in Geroscience and the Raymond and Margaret Labarge Chair in Research and Knowledge Application for Optimal Aging. The authors thank Meghan Kenny, Mary Gauld and Eva Tsakonis for their contribution to the relevance and quality assessment, and the data extraction phases; Maureen Rice for the search; Sharon Peck-Reid for database management and report formatting; Sarah Connor Gorber and Amanda Shane (Public Health Agency of Canada) for contributing to the original protocol development and reviewing drafts of the technical report; and the Child Obesity Working Group of the Canadian Task Force on Preventive Health Care (Patricia Parkin, Maria Bacchus, Neil Bell, Paula Brauer and Elizabeth Shaw) for providing comments on the protocol and initial analyses.
Footnotes
-
Contributors: Leslea Peirson, Donna Fitzpatrick-Lewis, Katherine Morrison, Rachel Warren and Parminder Raina were responsible for the study’s conceptualization, interpreting the data, and writing and revising the article. Leslea Peirson and Donna Fitzpatrick-Lewis were responsible for collecting the data and coordinating the project. Muhammad Usman Ali was responsible for analyzing the data, and writing and revising the article. All of the authors approved the final version of the manuscript submitted for publication and agree to act as guarantors of the work.
-
Funding: The Canadian Institutes of Health Research provided funding for this review but had no role in the design, analyses, interpretation or decision to submit the paper for publication.
References
- © 2015, 8872147 Canada Inc. or its licensors