Abstract
Background: Ranitidine was the most prescribed histamine-2 receptor antagonist (H2RA) in Canada when recalled in 2019 because of potential carcinogenicity. We sought to compare geographic and temporal patterns in use of prescription ranitidine and 3 other HRAs and estimated population exposure to ranitidine in 6 provinces between 1996 and 2019.
Methods: This population-based serial cross-sectional study used prescription claims for H2RAs dispensed from community pharmacies in Nova Scotia, Ontario, Manitoba, Saskatchewan, Alberta and British Columbia. We estimated the period prevalence of ranitidine use per 100 population by province, age category and sex. We estimated exposure to ranitidine between 2015 and 2019 using defined daily doses (DDDs).
Results: Overall, 2.4 million ranitidine prescriptions were dispensed to patients aged 65 years and older, and 1.7 million were dispensed to younger adults. Among older adults, the median period prevalence of ranitidine use among females was 16% (interquartile range [IQR] 13%–27%) higher than among males. Among younger adults, the median prevalence was 50% (IQR 37%–70%) higher among females. Among older adults, between 1996 and 1999, use was highest in Nova Scotia (33%) and Ontario (30%), lower in the prairies (Manitoba [18%], Saskatchewan [26%], Alberta [17%]) and lowest in BC (11%). By 2015–2019, use of ranitidine among older adults dropped by at least 50% in all provinces except BC. We estimate that at least 142 million DDDs of prescribed ranitidine were consumed annually in 6 provinces (2015–2019).
Interpretation: Over the 24-year period in 6 provinces, patients aged 65 years and older were dispensed 2.4 million prescriptions of ranitidine and younger adults were dispensed 1.7 million prescriptions of ranitidine. These estimates of ranitidine exposure can be used for planning studies of cancer risk and identifying target populations for cancer surveillance.
In September 2019, Health Canada stopped the distribution of ranitidine (a histamine-2 receptor antagonist [H2RA]) because of potentially high concentrations of N-nitrosodimethylamine (NDMA).1–4 This occurred subsequent to recalls in 2018 and 2019 for angiotensin receptor blockers called sartans based on observations that NDMA was a potent carcinogen in experimental animals and was classified as a probable human carcinogen.5,6
Before its recall, ranitidine was among the most commonly used drugs to treat gastroesophageal reflux disease (GERD). This disease is among the most prevalent gastroenterological disorders seen in clinical practice, affecting up to 20% of North American and European populations and less than 5% in east Asian populations.7 Symptoms of GERD — including dyspepsia (sensation of an upset or sour stomach), pyrosis (heartburn) and regurgitation — are among the most common complaints reported by people in Canada.8–10 Although GERD is not usually serious, the impact on patients is substantial; people with heartburn report bigger decrements to their health-related quality of life than patients with arthritis, diabetes, hypertension or mild heart failure.11 Consumed when needed to alleviate symptoms of GERD, prescription ranitidine first became available in Canada in 1982 with 3 other H2RAs (cimetidine, famotidine, nizatidine) marketed by 1988. After substantial deliberation, famotidine became available over the counter in 1996, followed by ranitidine in 1997 and cimetidine in 1998; nizatidine was never approved for over-the-counter sales.2,12,13 To date, limited contemporary population-based data are available on the use of ranitidine and other H2RAs in Canada. In this study, we sought to describe patterns of use of ranitidine and 3 other H2RA,s and exposure in 6 Canadian provinces between 1996 and 2019.
Methods
Setting and data sources
We conducted a population-based, multiprovince, descriptive study employing a serial cross-sectional design. We used data and analytic infrastructure maintained by the Canadian Network of Observational Drug Effect Studies (CNODES), a distributed network of investigators and linked databases that was established in 2011 to undertake collaborative, population-based studies of drug use, safety and effectiveness.14,15 We used prescription drug claims for H2RAs dispensed from community pharmacies in 6 Canadian provinces that make administrative health data available for researchers, namely Nova Scotia, Ontario, Manitoba, Saskatchewan, Alberta and British Columbia. Given differences in public drug insurance policies and data capture across provinces, data were available for all adults aged 18 years and older in BC, Alberta, Saskatchewan and Manitoba, and for those aged 65 years and older in Ontario and Nova Scotia. Data were unavailable or unobtainable in a timely manner in the other 4 provinces and 3 territories.
About 4 decades ago, many Canadian provinces began routinely collecting and collating claims on prescription drug dispensations, along with other types of administrative health data.16 These data are used to remunerate pharmacies for drugs dispensed from community pharmacies to all people in some provinces and to only those with public health insurance in other provinces. The drug dispensation claims identify the drug being dispensed, including the Drug Identification Number (indicating the drug, dosage form and strength) and the date, quantity and duration of the dispensation. Pharmacists must submit a claim to receive payment, and routine monitoring and measures are in place used to minimize misreporting. Once received, consistency checks are implemented to reduce coding errors. As such, drug dispensation claims have been shown to provide a valid and reliable representation of the drugs dispensed from community pharmacies because they are used for adjudicating payment, meaning that pharmacists are incentivized to submit the claims and the provincial drug plans have effective mechanisms to limit overpayment. 17 In Ontario, dispensation claims have been shown to have high coding reliability.18 Given that these processes are largely consistent across provinces and have not undergone major changes over time, these studies are likely highly representative of the reliability of drug dispensation claims from all provinces over the entire study period.
We included individuals if they received a dispensation claim for an H2RA between Jan. 1, 1996, and Dec. 31, 2019. Although data on dispensation claims were available in some provinces, 1996 was the first year in which these data were available in all participating provinces. We grouped H2RAs in Anatomic Therapeutic Chemical (ATC) classification system level 5, corresponding to ranitidine, famotidine, nizatidine or cimetidine, including tablet, capsule and oral liquid formulations. We excluded injectable formulations as they are typically administered to hospital inpatients and therefore unavailable in provincial drug claims data.
Data curation and analysis
We defined drug use using the period prevalence as a percentage of the population dispensed ranitidine or another H2RA in each of 5 calendar periods between 1996 and 2019 (a 4-year period, 1996–1999, followed by 5-year periods 2000–2004, 2005–2009, 2010–2014, 2015–2019). As the focus was on ranitidine because of concerns of exposure to a probable carcinogen, we grouped the other 3 H2RAs together for calculating the period prevalence. We identified people with at least 1 dispensation claim for ranitidine or any of the 3 other H2RAs within each calendar period. We counted those dispensed more than 1 type of H2RA in each stratum; those dispensed an H2RA in more than 1 calendar period were included more than once.19
For each province, the denominators for period prevalence rates were sex- and age-specific population estimates obtained from Statistics Canada.20 We estimated age- and sex-adjusted period prevalence rates using direct standardization. The reference population was the 2016 population of Canada, the most recent Census year that fell within the study period. To estimate exposure to ranitidine in the study populations in the most recent period (2015–2019), we estimated defined daily doses (DDDs) for all dispensation records of ranitidine, including refills and recurrent prescriptions, using reference values established by the World Health Organization Collaborating Center.21 Defined as “the assumed average maintenance dose per day for a drug used for its main indication in adults,”22 the DDD for ranitidine was 300 mg/d.23 Calculated by multiplying the dispensation quantity by the tablet strength and dividing by the DDD, these measures provide a fixed measure of prescribed drugs that is independent of prescription duration or strength. Applying DDDs makes it possible to compare drug utilization between jurisdictions and to develop sample size or power estimates for a population-based study of the association between ranitidine and the risk of gastrointestinal cancers and other purposes. For every dispensation claim for each recipient, we calculated the cumulative DDD for ranitidine by multiplying the quantity dispensed by the tablet strength and dividing by the DDD for ranitidine, as per the following formula, where k is the last dispensation in the calendar period:
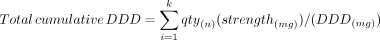
We tabulated the provincial mean DDDs across cumulative dispensations within each calendar period.
As the dispensation data available to CNODES sites includes information only on prescribed drugs, we obtained data on sales of over-the-counter ranitidine from IQVIA Canada, a contract research organization that curates and makes available data on over-the-counter sales of drugs sold in Canadian pharmacies (https://www.iqvia.com/locations/canada).24 The IQVIA data contain sales volumes from Canadian retail pharmacies for all branded and generic products.25 Nationally, more than 79% of total drugs are captured in a sample of about 6000 pharmacies. Monthly projections are thought to be representative of drug use at the provincial and national levels in Canada.26 The IQVIA quantity and tablet strength of over-the-counter sales of ranitidine sold in community pharmacies in all 6 provinces were not available for the entire study period so we obtained 2015–2019 data to estimate the cumulative DDDs in the most recent calendar period.
Given that this was a descriptive study,27,28 we sought to stratify the results by population descriptors including province, time, sex and age group. Although we stratified the results by these descriptors and directly standardized prevalence by age group, we did not seek to make inferences to a larger population defined in time or by province, age or sex. As such, we report prevalence and DDDs without associated confidence intervals.
Ethics approval
We obtained research ethics board approvals from each participating institution except for ICES (in Ontario, where research ethics board approval was not legally required). Ethics approval was obtained at the Health Research Ethics Board of Alberta at the University of Calgary, the Clinical Research Ethics Board at the University of British Columbia, the Health Research Ethics Board at the University of Manitoba, the Health Sciences Research Ethics Board at Dalhousie University in Nova Scotia and the Biomedical Research Ethics Board at the University of Saskatchewan.
Results
Table 1 shows the number of people who received at least 1 dispensation for ranitidine or another H2RA, along with the population denominator, in 6 Canadian provinces by age stratum and calendar period. Over the 24-year study period, people aged 65 years and older were dispensed 2.4 million prescriptions of ranitidine and 0.7 million prescriptions of any other H2RA; those aged 18–64 years were dispensed 1.7 million prescriptions of ranitidine and 0.6 million prescriptions of another H2RA.
Number of people dispensed at least 1 type 2 histamine-2 receptor antagonist (H2RA) in 6 Canadian provinces and sex-standardized period prevalence rates per 100 population, by age stratum and calendar period, 1996–2019
Table 1 also shows the sex- and age-standardized period prevalence of ranitidine and 3 other H2RAs in 5 calendar periods between 1996 and 2019 for people aged 65 years and older and those younger than 65 years. Among those aged 65 years and older, geographic trends observed in the first period (1996–1999) were that the prevalence of use of all H2RAs was highest in Ontario and lowest in Manitoba and Alberta; use of ranitidine was about threefold higher than the 3 other H2RAs in all provinces except BC, where the 3 other H2RAs were used about twice as often as ranitidine; and the prevalence of ranitidine use followed a rough East–West gradient (stronger in the first 2 calendar periods), with rates of being dispensed an H2RA highest in Nova Scotia and Ontario (39%–42%) and lower in the Western provinces (25%–35%).
In provinces that included dispensation data for people younger than 65 years, the period prevalence of all 4 H2RAs combined was between 40% and 60% of that observed in the older age category. In this age group, geographic trends observed in the first period (1996–1999) were that prevalence of use of all H2RAs was similar in the 3 provinces contributing data for all residents (Manitoba, Saskatchewan and BC) and use of ranitidine was about twice as high as that of other H2RAs in all provinces except BC, where use of the 3 other H2RAs was slightly higher than use of ranitidine.
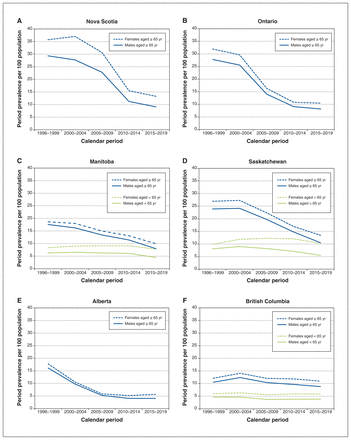
Figure 1 shows sex-specific period prevalence rates of prescription ranitidine in the 6 Canadian provinces over 5 calendar periods between 1996 and 2019, including the 3 provinces that collected prescription claims data on people aged 65 years and older for the entire period (Nova Scotia, Ontario, Alberta) and 3 provinces also including people younger than 65 years (Manitoba, Saskatchewan, BC). Among people aged 65 years and older, ranitidine use peaked in 1996–1999 or 2000–2004 and declined thereafter. By the last period, the prevalence was about 40%–60% lower than the peak for all provinces except BC (which had a smaller decline, given lower rates in the earlier periods). Among people younger than 65 years, ranitidine use declined slightly in Saskatchewan and remained consistent in Manitoba and BC.
Sex-specific period prevalence rates (per 100 population) of prescription ranitidine in (A) Nova Scotia, (B) Ontario, (C) Manitoba, (D) Saskatchewan, (E) Alberta and (F) British Columbia, by age stratum and calendar period, 1996–2019.
Across all 6 provinces and in both age categories, Figure 1 consistently shows that females were more commonly dispensed an H2RA than males, with a median rate of 16% (interquartile range [IQR] 13%–27%) higher among females aged 65 years and older and 50% (IQR 37%–70%) higher among females younger than 65 years. The temporal trends in use were effectively parallel between the sexes.
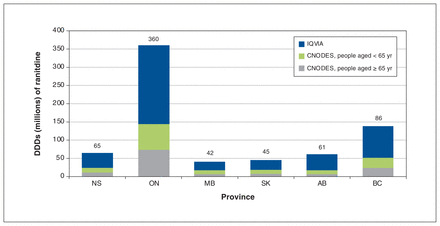
Figure 2 presents the estimated cumulative DDDs of prescribed ranitidine dispensed in the 6 Canadian provinces between 2015 and 2019, including data on over-the-counter sales. With data imputed for those younger than 65 years in Nova Scotia and Ontario, combined with the mean per capita DDDs in Manitoba, Saskatchewan and BC, we estimate that a minimum of 142 million DDDs of prescribed ranitidine were consumed annually in all 6 provinces.
Total number of ranitidine defined daily doses (DDDs) dispensed from community pharmacies (source: Canadian Network of Observational Drug Effect Studies [CNODES]) or purchased over the counter (source: IQVIA) in Nova Scotia (NS), Ontario (ON), Manitoba (MB), Saskatchewan (SK), Alberta (AB) and British Columbia (BC), 2015–2019. Number of DDDs for people younger than 65 years in NS and ON were extrapolated by applying the mean proportion (24.5%) of over-the-counter sales in that age stratum in MB, SK and BC. Excludes prescription ranitidine dispensed in hospitals and from pharmacies when the payer was not the government.
Interpretation
Use of H2RAs increased rapidly after coming to market in the early 1980s, likely because they filled an unmet health need for many people with GERD, along with favourable efficacy and safety profiles. Use of H2RAs then dropped after approval of proton pump inhibitors (PPIs) in the late 1980s because PPIs produce greater acid suppression than H2RAs.8,29 By 1995, the suggested approaches to treating GERD included single-agent therapy, step-up therapy and step-down therapy, with step-up therapy using H2RAs being encouraged in primary care.30 In this study, we observed that, beginning in 1996, ranitidine was the most commonly used H2RA in the populations in all included provinces except BC where, in the first 2 calendar periods, reference-based pricing was implemented with generic cimetidine.31
For most provinces, the period prevalence for use of H2RAs for both sexes peaked within the first 2 calendar periods and then declined. This pattern can be explained by the increased sales of PPIs,32–37 which became available in Canada in 1989 and which produce greater acid suppression than H2RAs and exhibit better efficacy for GERD and other gastrointestinal indications.8,29,38 It seems likely that increasing use of PPIs displaced the use of H2RAs after 2005. The period prevalences did not seem to depend on whether the period was 4 years (1996–1999) or 5 years, perhaps because the duration was relatively long.
Describing the epidemiology of GERD requires consideration of whether the occurrence and health services differ by sex or gender.39 Although some studies have shown higher rates of GERD among women than men,40 others have observed no difference between the sexes.41 More severe manifestations of erosive disease, notably Barrett’s esophagus, are more commonly observed among males.42–44 Higher rates of invasive diagnostic testing among females in the United States were attributed more to differences in health-seeking behaviour and socioeconomic factors than the biology of GERD.45 In Pennsylvania, females with GERD were almost twice as likely to be referred for ambulatory testing.46 In the current study, females had higher ranitidine use, which could be related to sex differences in the natural history of GERD,40 health-seeking behaviour47 or differences in prescribing.48 However, the striking parallels in the temporal changes in ranitidine use provide evidence of biological differences by sex in the epidemiology of GERD. Greater understanding of sex differences in prescribing is important from both clinical39,40,49 and policy50 perspectives.
Overall, there remains limited descriptive data characterizing use of prescription ranitidine and other H2RAs in Canada and in other jurisdictions. Three studies used service claim information to characterize utilization trends.51–53 Tett and colleagues52 compared use of H2RAs and PPIs among older adults and social security beneficiaries in Australia and Nova Scotia between 2001 and 2005 using data from the Australian Pharmaceutical Benefits Scheme and Nova Scotia Pharmacare. In Nova Scotia, just over 185 000 eligible beneficiaries were identified in the 2003/4 year and 4.95 million concession beneficiaries were identified in Australia in 2005.52 Those authors found that prescription ranitidine was the most common H2RA used in both countries (up to 95% in Nova Scotia and up to 82% in Australia). Yu and colleagues53 observed that, between 2004 and 2017, mean annual use of H2RAs declined in Australia (−5.1%) and PPI use increased in the Republic of Korea (+14%). Several studies evaluated the impact of policy and interventions on prescribing patterns in Belgium,54 BC31 and the US.55,56 The implementation of reference-based pricing and special authority–restricted reimbursement of PPIs for older adults receiving BC Pharmacare was associated with a substantial increase in mean monthly prescriptions of cimetidine (379%) and reduction in prescriptions of restricted H2RAs (55%).31
Although NDMA is classified as a probable carcinogen by the International Agency for Research on Cancer, the epidemiologic evidence has been equivocal, with a statistically significant association between ranitidine and gastrointestinal cancers documented by some investigators (e.g., Wang and colleagues57) and not by others (e.g., Yoon and colleagues58). In this case, it is important to distinguish between studies that are rigorously designed with sufficient exposed follow-up time, powered to rule out an association if none exists. This issue is complicated because the amount of NDMA in ranitidine can increase during storage, depending, among other things, on the storage temperature. The follow-up spanning decades and high numbers of Canadians potentially exposed to NDMA from ranitidine described herein could form the basis of a very large pharmacoepidemiologic study on the putative association. Such a study would face challenging methodological hurdles of estimating exposure to ranitidine when the drug was used as needed and was available both prescribed and over the counter.
The main strength of this study was the standardization of the design and analysis through a single protocol for all carefully curated data from 6 provincial data repositories, minimizing information biases and likely rendering these biases nondifferential across provinces. As this population-based review of drug use in 6 Canadian provinces between 1996 and 2019 included standardly collected and curated drug dispensation data, use of prescription ranitidine and other prescription H2RAs is likely accurate over the study period.
Limitations
Several limitations led to underestimates of the national use and exposure to ranitidine and other H2RAs. Although our study used population-based data of eligible people in participating provinces, using secondary sources of data meant that large segments of the Canadian population were not included because they did not have provincial drug coverage, including those younger than 65 years in Nova Scotia and Ontario, or were residents of other provinces and territories (New Brunswick, Newfoundland and Labrador, Northwest Territories, Nunavut, Prince Edward Island, Quebec, Yukon Territories). IQVIA data do not include all over-the-counter sales, thus underestimating the exposure to ranitidine in the population. Over-the-counter sales predated the availability of IQVIA data.
Conclusion
This study quantifies the large number of people in Canada exposed to prescription ranitidine over 24 years. The estimated cumulative ranitidine DDDs, highest in Ontario among people older than 65 years and highest in BC among younger adults, provide targets for cancer surveillance. Additional considerations for surveillance are the increased incidence and mortality rates observed in eastern Canada for gastric cancers and for cancers combined. Given the limited human data characterizing the relationship between nitrosamine exposure and cancer risk, the population exposure to ranitidine can also inform a pharmacoepidemiologic study of cancer risk in Canada in which administrative health data on drug dispensation claims are linked with data from provincial cancer registries.
Acknowledgements
The authors acknowledge the contributions of Fangyun Wu (Ontario), Xinya Lu (Saskatchewan), Jordan Hunt (Canadian Institute for Health Information), Kathy Lee (Canadian Institute for Health Information), Carolina Moriello (Quebec) and Kristian Filion (Quebec). This study was made possible through data-sharing agreements between CNODES member research centres and the respective provincial governments of Alberta, British Columbia, Manitoba, Nova Scotia, Ontario and Saskatchewan.
Footnotes
Competing interests: Laura Targownik has served on advisory boards or created educational materials for Janssen AbbVie Canada, Takeda Canada, Merck Canada, Pfizer Canada, Janssen Canada, Roche Canada, Sandoz Canada, JAMP Pharmaceuticals and Celltrion Pharmaceuticals. No other competing interests were declared.
This article has been peer reviewed.
The Canadian Network for Observational Drug Effect Studies (CNODES) Investigators: Samy Suissa, Colin R. Dormuth, Brenda R. Hemmelgarn, Jacqueline Quail, Dan Chateau, J. Michael Paterson, Jacques LeLorier, Adrian R. Levy, Pierre Ernst and Kristian B. Filion, Lisa M. Lix, Robert W. Platt and Ingrid S. Sketris
Contributors: Adrian Levy, David Stock, J. Michael Paterson, Hala Tamim and Laura Targownik contributed to the conception of the work. All of the authors contributed to data acquisition, analysis and interpretation. Adrian Levy, David Stock, J. Michael Paterson, Hala Tamim and Laura Targownik drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: The Canadian Network for Observational Drug Effect Studies, a collaborating centre of the Drug Safety and Effectiveness Network (DSEN), is funded by the Canadian Institutes of Health Research (Grant Number DSE-146021).
Data sharing: Data for the study may be accessed through the data repositories in each of the 6 included provinces.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/11/6/E1033/suppl/DC1.
Disclaimer: This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and Ministry of Long-Term Care (MLTC). The study used data adapted from the Statistics Canada Postal CodeOM Conversion File, which is based on data licensed from Canada Post Corporation, and/or data adapted from the Ontario Ministry of Health Postal CodeOM Conversion File, which contains data copied under license from Canada Post Corporation and Statistics Canada. Parts of this material are based on data and/or information compiled and provided by the Ontario Ministry of Health and the Canadian Institute for Health Information (CIHI). The authors thank IQVIA Solutions Canada for use of their Drug Information File. The authors also acknowledge the Manitoba Centre for Health Policy for use of data contained in the Manitoba Population Research Data Repository under project, HIPC # 2020/2021–53. Data used in this study are from the Manitoba Population Research Data Repository housed at the Manitoba Centre for Health Policy, University of Manitoba, and were derived from data provided by Manitoba. The BC Ministry of Health approved access to and use of BC data facilitated by Population Data BC for this study. This study is based in part on data provided by Alberta Health. The opinions, results and conclusions reported in this paper are those of the authors and do not reflect the opinions or policies of the Data Stewards. No endorsement by the funders, data providers, Data Stewards, the Governments of Alberta, British Columbia, Manitoba, Nova Scotia, Ontario and Saskatchewan, ICES, the Manitoba Centre for Health Policy, Health Canada, CIHI or Canadian Institutes of Health Research is intended or should be inferred. The opinions, results and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOH or MLTC is intended or should be inferred.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/
References
- © 2023 CMA Impact Inc. or its licensors