Abstract
Background: Despite increases in cases of Lyme disease, little is known about the management and clinical course of the disease in Canada. We aimed to describe the management and clinical course of Lyme disease in patients treated in acute care facilities in Quebec and to assess adherence to the 2006 Infectious Diseases Society of America (IDSA) guideline.
Methods: This retrospective multicentre cohort study included pediatric and adult patients with serologically confirmed Lyme disease treated in acute care facilities (12 community hospitals and 2 tertiary care centres) of 2 endemic regions of Quebec (Estrie and Montérégie), from 2004 to 2017. We considered drug choice, prescribed dose and treatment duration in assessing adherence of prescriptions to the 2006 IDSA guideline. The main outcome was complete resolution of symptoms at 3 months after the initiation of treatment.
Results: We included 272 patients from 14 institutions (age range 3–87 yr). Early disseminated Lyme disease (140 patients [51%]) was predominant. Adherence to the IDSA guideline was observed in 235 (90%) of the 261 cases with complete information, and adherence was stable over time (2004–2013: 57/64 [89%]; 2014–2015: 64/71 [90%]; 2016–2017: 114/126 [90%]; p = 0.8). Non-adherence to the guideline (n = 26) was predominantly due to longer-than-recommended treatment duration (16/26 [62%]). Resolution of objective signs at 3 months after treatment initiation occurred in 265 (99%) of 267 patients, whereas post-treatment Lyme disease syndrome was observed in 27 patients (10%) with increasing incidence over time (2004–2013: 3/65 [5%]; 2014–2015: 4/73 [5%]; 2016–2017: 20/129 [16%]; p = 0.02).
Interpretation: We observed clinical resolution of Lyme disease in 99% of the patients, and most treatments (90%) complied with the 2006 IDSA guideline. The incidence of post-treatment Lyme disease syndrome increased over the study period, warranting further prospective studies.
Lyme disease, a multisystem infection primarily caused by Borrelia burgdorferi in North America and by Borrelia afzelii and Borrelia garinii in Europe and Asia,1,2 progresses in 3 stages: early localized stage, early disseminated stage and late disseminated stage.3 Cases of Lyme disease reported in Canada increased from 144 in 2009 to 992 in 2016, representing an increase from 0.4 to 2.7 per 100 000 population.4
In Quebec, Lyme disease has been notifiable since November 2003, with the first locally acquired case reported in 2006.5,6 Reported cases of Lyme disease and the proportion of cases with acquired infection have increased each year.6 In 2017, 329 cases were declared to the public health authorities in Quebec, including 249 (76%) acquired in that province, particularly in Estrie (n = 138, 55% of Quebec-acquired cases) and Montérégie (n = 75, 30% of Quebec-acquired cases).6
Despite the increase in cases, little is known about the management and clinical course of Lyme disease in Canada. Published evidence has focused on epidemiologic surveillance, risk of acquisition and clinical case characteristics.7–9 We aimed to describe case management of Lyme disease in acute care facilities in Quebec and adherence to the 2006 guideline of the Infectious Diseases Society of America (IDSA).3 We assessed the clinical course of patients treated in Quebec and temporal changes in case severity from 2004 to 2017.
Methods
Study design and setting
We conducted a retrospective cohort study in all 14 acute care facilities in the Estrie and Montérégie regions of Quebec, Canada, which in 2019 had populations of 489 479 and 1 421 586, respectively.10 These regions account for most cases of Lyme disease in Quebec.11 These acute care facilities consist of 12 community hospitals and 2 tertiary care centres, which also receive transfers from outside these regions. This study was reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline checklist for cohort studies.12
Population
The study population consisted of all pediatric and adult patients with serologically confirmed Lyme disease as per the 2-tiered testing algorithm currently used in Canada13 and treated (whether in emergency departments, as inpatients or as outpatients) in 1 of the 14 acute care facilities in the study regions. The 2-tier serologic testing algorithm involves an enzyme immunoassay followed, where appropriate, by confirmatory Western blots using diagnostic kits licensed in Canada. During the study period, all enzyme immunoassays were performed at the Quebec public health laboratory, with final confirmation by immunoblot at the National Microbiology Laboratory in Winnipeg.
We excluded patients seen in the offices of primary care physicians, because their medical records were not available. We also excluded patients whose medical records were not available for other reasons or for whom the data needed for the study (e.g., clinical information needed to assess clinical stage) were incomplete.
Data sources
We examined patient charts at the 14 acute care centres. Six research assistants performed these chart reviews using a standardized data abstraction form (Appendix 1, available at www.cmajopen.ca/content/10/2/E570/suppl/DC1). This form was developed by 3 members of the research team (J.B.M., D.M., A.C.) after an initial review of the literature, 1,3,5,7 and was pretested on 20 patients. All research assistants were trained, and a data collection guide (Appendix 2, available at www.cmajopen.ca/content/10/2/E570/suppl/DC1) was developed to ensure accuracy in data collection. A primary care physician (J.B.M.) and a research coordinator (D.M.) with expertise in Lyme disease verified the completed forms before data entry. We did not assess inter-rater reliability.
We collected medical history data to calculate the Charlson Comorbidity Index,14 along with demographic, microbiologic and therapeutic data. We also recorded data on clinical signs and symptoms present before treatment and at 3 and 12 months after the initial treatment. We also documented whether the patient had been referred to a specialist.
We extracted data on patients’ serologic status from a database at the Laboratoire de santé publique du Québec, where all results of serology testing for Lyme disease are centralized for the province of Quebec. Immunoblot results from the National Microbiology Laboratory in Winnipeg are also included in this database. We extracted these data by selecting cases with positive results on serology or polymerase chain reaction testing. We considered only patients living in the 2 regions of interest (Estrie and Montérégie). A clinical specialist in medical biology (K.T.) performed the data extraction.
We also extracted data from the regional registries for notifiable diseases to assess potential changes in the proportion of serologically confirmed cases of Lyme disease managed in acute care facilities relative to those treated in community settings.
Outcomes
The main outcome was the proportion of patients with clinical resolution of Lyme disease at 90 days, defined as the complete disappearance of objective clinical signs found on physical examination (e.g., facial palsy, synovitis).3 We defined treatment failure as persistence of at least 1 objective sign 90 days after the initial treatment.3 We did not consider subjective symptoms, such as fatigue or arthralgia, in our evaluation of the primary outcome. The secondary outcome was the presence of post-treatment Lyme disease syndrome, defined as the presence of any of the following symptoms: widespread musculoskeletal pain, cognitive complaints, radicular pain, paresthesia or dysesthesia.3 The symptoms also had to begin within 6 months after the initial diagnosis and treatment of B. burgdorferi infection and had to persist for at least 6 months.3
We formed an adjudication committee consisting of 1 primary care physician with expertise in Lyme disease (J.B.M.), 2 infectious diseases fellows (S.B.D. and A.A.P.) and an infectious disease consultant (A.C.), who independently assessed the clinical stage of each case of Lyme disease, adherence of the prescribed treatment to the IDSA guideline and the outcomes; the infectious disease consultant also served as chair-person of the committee. We defined clinical stages according to the IDSA guideline.3 In case of disagreements among adjudicators, consensus meetings were held for resolution.
We assessed the adherence of physician prescribing to the IDSA guideline according to the clinical stage of each patient, the treatment given, the drug dose and the treatment duration (Table 1). After reviewing the literature, we chose to compare the management of patients in our cohort to this guideline, issued in 2006, as it was used as a reference in North America, was relevant during most of our study period and was endorsed in 2019 by the Association of Medical Microbiology and Infectious Disease Canada.15 The 2006 guideline replaced the “Practice Guidelines for the Treatment of Lyme Disease” issued by IDSA in 2000.16 Because we wanted to document changes in practice, we assessed all cases in the study, including those from 2004 and 2005, against the 2006 guideline, to ensure that all patients were assessed according to the same criteria. Nonadherence with any of the 3 criteria was considered to represent nonadherence of the physician’s prescription to the guideline.
Summary of the 2006 Infectious Diseases Society of America guideline for treatment of Lyme disease3
To assess potential changes in the presentation of Lyme disease, we divided the overall study period into 3 periods according to the incidence of Lyme disease in Quebec: 2004–2013, when incidence was low; 2014–2015, when incidence was moderate; and 2016–2017, when incidence was high.6 These periods were chosen to ensure a similar number of cases in all periods. We used regional notifiable disease registries to assess potential changes in the proportion of serologically confirmed cases of Lyme disease managed in acute care facilities.
Statistical analysis
We double-entered the data into an electronic input tool (Research Electronic Data Capture [REDCap], Vanderbilt University) and analyzed them using Stata 15.1 for Mac (Stata-Corp). We compared proportions of characteristics between study periods using the χ2 test or the Fisher exact test, as appropriate. We compared continuous variables using the Wilcoxon rank-sum test or the analysis of variance test, as appropriate.
Ethics approval
The institutional review board of the Centre intégré universitaire de santé et de services sociaux de l’Estrie – Centre hospitalier universitaire de Sherbrooke approved this study (project MP-31–2020–3251).
Results
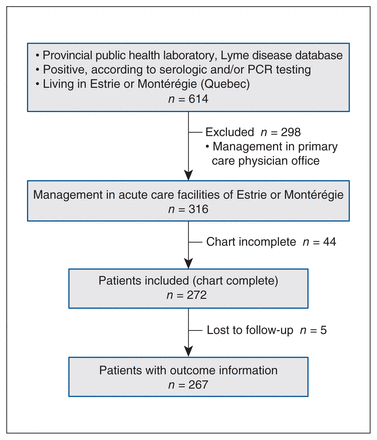
A flow chart detailing the study inclusion and exclusion steps is shown in Figure 1. Overall, we extracted 1114 positive results on serology or polymerase chain reaction tests (or both) from the public health laboratory database. Of these, 316 patients lived in the Estrie and Montérégie regions and their care was managed in acute care facilities of these regions between 2004 and 2017. Among these patients, 272 cases (86%) had complete medical records analyzed. Of these, 252 (93%) had acquired Lyme disease in North America and 3 in Europe; the acquisition site was unknown for 17 patients.
Study flowchart. Note: PCR = polymerase chain reaction.
The baseline characteristics of the cases are summarized in Table 2. Most patients were male (169/272; 62%). Patients ranged in age from 3 to 87 years, with median age 51.5 years (interquartile range [IQR] 32.6–62.5). Most patients had no comorbidities, indicated by a Charlson score of 0 for 222 (82%) of the patients. Only 19 patients (7%) had substantial immunosuppression. To investigate potential selection bias, we compared the included patients with those excluded because of incomplete data in terms of their baseline characteristics, but found no significant differences (Appendix 3, available at www.cmajopen.ca/content/10/2/E570/suppl/DC1).
Characteristics of patients with Lyme disease according to study periods
The early disseminated stage of Lyme disease (n = 140; 51%) predominated in our cohort, followed by the localized early stage (n = 90; 33%) and late disseminated stage (n = 42; 15%). Of the 140 patients with the early disseminated stage, 88 (63%) had multiple erythema migrans, 40 (29%) had facial palsy, 30 (21%) had meningitis and 9 (6%) had radiculopathy. Among these patients, cardiac involvement was in the form of carditis (n = 8; 6%), first-degree atrioventricular block (n = 4; 3%), second-degree atrioventricular block (n = 3; 2%) and third-degree atrioventricular block (n = 4; 3%). Among cases of the late disseminated stage, most patients (41/42; 98%) had arthritis.
A minority of the 272 patients received care from primary care or emergency physicians only (n = 50; 18%); 193 (71%) were referred to infectious disease specialists, and 17 (6%) were referred to neurologists. Forty-eight patients required hospitalization related to Lyme disease within 3 months of diagnosis, with a median duration of 4 (IQR 1–7) days, including 7 patients admitted to an intensive care unit (median duration 1 [IQR 1–4] d). One death unrelated to Lyme disease occurred, at 589 days after diagnosis.
Treatment and adherence to the IDSA guideline
Antimicrobial treatment included doxycycline (n = 171; 63%), amoxicillin or amoxicillin–clavulanic acid (n = 39; 14%) and ceftriaxone (n = 46; 17%). Antibiotic treatment was started before the final immunoblot result was issued in 216 patients (79%) and after the final immunoblot result was issued in the remaining 56 patients (21%). Most patients stated that they had completed the prescribed treatment regimen (258/266; 97%).
We assessed adherence of the physician’s prescription to the IDSA guideline for the 261 (96%) patients for whom complete information on the antibiotic treatment regimen was available. Of these patients, 235 (90%) received a treatment supported by the IDSA guideline. Nonadherence of the physician’s prescription to the guideline was due to a longer-than-recommended (16/26; 62%) or shorter-than-recommended (8/26; 31%) treatment duration, use of a nonsupported antimicrobial (8/26; 31%) or use of an antimicrobial dosage lower than suggested by the guideline (1/26; 4%), with some prescriptions having more than 1 problem. Among patients with a longer-than-recommended treatment duration, the median treatment duration was 28 (IQR 27–30) days, in contrast to 20 days among patients with the guideline-recommended treatment duration.
Outcome assessment
Of the 272 patients included in the study, 5 were lost to follow-up; as such, the medical charts had enough data to allow assessment of primary and secondary outcomes for 267 (98%) of the patients. We observed complete resolution of objective signs of infection in 265 (99%) of these 267 patients at 90 days. We documented post-treatment Lyme disease syndrome in 27 patients (10%). Factors associated with this syndrome in univariate analyses are shown in Table 3.
Characteristics of patients with and without post-treatment Lyme disease syndrome (PTLDS)
Temporal changes in characteristics, treatment and outcomes
To investigate temporal changes in the presentation of Lyme disease further, we compared the characteristics of cases diagnosed between 2004 and 2013 (low-incidence period; n = 66), in 2014 and 2015 (moderate-incidence period; n = 74), and in 2016 and 2017 (high-incidence period; n = 132) (Table 2).
Among all patients in the regional registries with a positive result, the proportion seen in acute care facilities (v. community settings) decreased significantly over the study period (2004–2013: 66/122, 54%; 2014–2015: 74/181, 41%; 2016–2017: 132/358, 37%; p < 0.001). Adherence to the IDSA guideline remained stable over time. There was a significant increase over the 3 incidence periods in the proportion of patients with development of post-treatment Lyme disease syndrome (p for trend = 0.02).
Interpretation
Most patients with Lyme disease who were treated in 14 acute care institutions of 2 endemic regions of Quebec and who experienced complete resolution of objective clinical signs following their treatment had received antimicrobial therapy in accordance with the IDSA guideline. Given that our sample included all acute care institutions within these 2 regions, we were able to assess most of the more severe cases encountered within these regions.
These results are reassuring, in a context where concerns about the management of Lyme disease have recently been raised at the public and political levels in Quebec.17
In more than 99% of cases in our study, the clinical signs completely disappeared within 3 months (90 days) of treatment initiation. Our results are similar to those of 2 other studies conducted in North America, which showed complete resolution of clinical signs in 99.3%18 and 90%19 of patients. Therefore, the treatment applied seems effective in eliminating incapacitating clinical signs, such as Bell palsy. However, other studies conducted in pediatric and adult patients have found lower rates of clinical resolution.20,21 This discrepancy may be explained by the fact that these latter studies included mainly cases of Lyme arthritis, whereas in our study the proportion of patients with arthritis was only 15% (41/272).
Although most patients in our study responded very well to treatment, 10% of the patients experienced post-treatment Lyme disease syndrome. These results are in line with other studies, which have reported similar proportions of patients with this syndrome.18,19,22 They also underline the necessity of conducting prospective studies to gain a better understanding of both the causes and treatment options for patients with post-treatment Lyme disease syndrome, a condition that is distressing for affected patients.23
Since 2004, the turnaround time for diagnosis of Lyme disease in Quebec has remained stable, at almost 40 days. For many years, most provincial public health or hospital laboratories have performed enzyme immunoassay testing locally, while immunoblot testing was performed at the National Microbiology Laboratory in Winnipeg. The implementation of immunoblot testing in provincial public health laboratories might help to decrease these turnaround times. The implementation of a modified 2-tier test system, in which a second enzyme immunoassay is used, instead of the traditional immunoblot, might also result in diagnostic improvements in the sensitivity of testing for Lyme disease.24
We observed overall adherence of 90% to the IDSA guideline for treatment of Lyme disease, lower than adherence rates previously published in other contexts. For example, an adherence rate of 100% was reported for a small sample of patients with erythema migrans; however, in that study, the criteria for guideline adherence were not specified, and treatment information was available for only 25% of the reported cases.25 Another study conducted in a hyper-endemic area of Wisconsin showed treatment adherence with IDSA recommendations in 99.9% of cases,19 but it included only cases of early-stage Lyme disease. Furthermore, the only criterion employed for comparing treatment with the IDSA guideline was the type of antimicrobial used. We considered more stringent comparison criteria, whereby treatments deemed adequate had to involve the right molecule, at the right dosage, for an adequate duration, which might explain our lower adherence rate.
Our population was restricted to patients receiving care in acute care facilities, which might have reflected more complex cases than those seen in primary care practices. In cases of nonadherence to the IDSA guideline, the main reason identified was a longer treatment duration. Given that prolonged antimicrobial therapy for the treatment of Lyme disease has not shown greater efficacy than shorter-duration regimens, 2,18,26 it is important to educate clinicians about the dangers of prolonged treatment, which may lead to significant risks, such as Clostridioides difficile colitis.27 The 2020 IDSA guideline also emphasized reducing as much as possible the duration of antibiotic therapy to reduce potential antibiotic-associated complications.28
We found that the proportion of patients with serologically confirmed Lyme disease who were managed in acute care facilities, a complex population, decreased significantly over time in the regions we studied. Studies in North America have found a gradual increase in the proportion of cases in the early localized stage, which consequently implies a gradual decrease in the proportion of cases with severe Lyme disease.7,29–31 Over time, clinicians are becoming more familiar with Lyme disease and hence can diagnose and treat the disease early, thus preventing the passage to more advanced stages.
Limitations
The main limitation of our study was its retrospective nature, which could introduce information bias. We minimized this type of bias by involving specialized research assistants who ensured complete data collection.
A total of 44 patients were excluded from the analysis because of incomplete information in the chart. Given that we did not have access to clinical information for these patients, we could not assess the potential bias that might have been introduced by these exclusions. However, the demographic characteristics of the included and excluded patients were similar, so we believe it is unlikely that this caused selection bias.
We had limited power to confirm several indicators of increasing awareness and familiarity with Lyme disease in both patients (decreasing time to the first consultation) and clinicians (decreasing time between first symptoms and treatment initiation). Also, we could not perform multivariable modelling regarding risk factors for post-treatment Lyme disease syndrome because of an insufficient number of events.
For purposes of our analysis, we assessed the earliest cases in this study against guideline recommendations that had not yet been published at the time the patients received care.
Conclusion
In this retrospective multicentre cohort study in 2 endemic regions of Quebec, we observed resolution of the objective signs of Lyme disease in most patients and found that most treatments were adherent with the 2006 IDSA guideline. We have also described the emergence of post-treatment Lyme disease syndrome, a finding that warrants further investigation in prospective studies.
Footnotes
Competing interests: For work unrelated to the current study, Emmanuelle Cantin has received grants from the Centre de recherche du CHU Sherbrooke. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Jean Musonera, Louis Valiquette, Geneviève Baron, Dominique Marcoux and Alex Carignan conceived and designed the study. Louis Valiquette, Geneviève Baron, François Milord, Dominique Marcoux, Karine Thivierge, Samuel Bedard-Dallaire, Andrée Pelletier, Raphaël Lachance, Jeremy Bourget, Catherine Simard, Emmanuelle Cantin, Farhad Abbasi, Louis-Patrick Haraoui and Alex Carignan participated in the acquisition, analysis and interpretation of the data. Jean Musonera, Louis Valiquette, Geneviève Baron, François Milord, Karine Thivierge, Louis-Patrick Haraoui and Alex Carignan wrote the original draft, and all of the authors revised the manuscript for important intellectual content. All of the authors approved the final version for publication and agreed to be accountable for the work.
Funding: This work was supported by the Centre de recherche du CHU Sherbrooke.
Data sharing: The data that support the findings of this study are available from the corresponding author (A.C.) upon reasonable request.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/10/2/E570/suppl/DC1.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/
References
- © 2022 CMA Impact Inc. or its licensors