Abstract
Background: We evaluated the impact of publicly funded pharmacare (Ontario Health Insurance Plan [OHIP]+), which was introduced in Ontario on Jan. 1, 2018, for youth less than 25 years of age, on temporal trends in hemoglobin A1c (HbA1c, a measure of glycemic management) and the differential effect on the change in temporal trends in HbA1c according to socioeconomic status (SES).
Methods: We conducted a trend analysis using administrative data sets. We included youth aged 21 years, 9 months or younger, residing in Ontario on Jan. 1, 2016, with diabetes diagnosed before age 15 years and before Jan. 1, 2015. We used claims for insulin to measure pharmacare use. We evaluated the change in HbA1c (%) per 90 days before (Jan. 1, 2016, to Dec. 31, 2017) the introduction of and during (Apr. 1, 2018, to Mar. 31, 2019) OHIP+ coverage, and the difference in the change in HbA1c according to SES, using segmented regression analysis.
Results: Of 9641 patients, 7041 (73.0%) made an insulin claim. We found a negligible difference in the temporal change in HbA1c during compared with before OHIP+ coverage that was not statistically significant (β estimate −0.0002, 95% confidence interval [CI] −0.0004 to 0.0000). The size of the effect was slightly greater in those individuals with the lowest SES than in those with the highest SES (β estimate −0.0008, 95% CI −0.0015 to −0.0001).
Interpretation: We found that the effect of OHIP+ on the change in HbA1c was slightly greater for youth in the lowest SES than for those in the highest SES. Our findings suggest that publicly funded pharmacare may be an effective policy tool to combat worsening socioeconomic disparities in diabetes care and outcomes.
Type 1 diabetes is a common chronic disease of childhood with substantial morbidity and mortality. 1 Optimizing glycemic management is key to preventing complications.2,3 There are known socioeconomic status (SES) disparities in diabetes management and outcomes.4,5 For Canadians, financial barriers to medications continue to be an important adverse social determinant of health.6 This is important because insulin is life sustaining for people with type 1 diabetes.
Ontario residents (population 14.5 million) have publicly funded coverage for medically necessary services but not for prescription medications. On Jan. 1, 2018, the Ontario government introduced publicly funded pharmacare (Ontario Health Insurance Plan [OHIP]+), the first payer for drugs for Ontarians younger than 25 years.
Before the introduction of OHIP+, only people older than 64 years and those eligible for social assistance had access to publicly funded medications; all others paid out of pocket, through private insurance or were supported, in part, by the Trillium Drug Program (a government program that provides drug coverage for out-of-pocket expenses that are more than 4% of household income). The OHIP+ program covered the cost of medications on the provincial formulary, with no deductible or copayment. Fifteen months after its introduction, the Ontario government changed OHIP+ to exclude those patients with private drug coverage.7 Thus, Ontario provides a unique “natural experiment” to assess the impact of 15 months of publicly funded pharmacare.
Our objective was to determine the impact of publicly funded pharmacare for youth younger than 25 years with type 1 diabetes on the temporal trend in hemoglobin A1c (HbA1c), a proxy for the risk of long-term diabetes complications. 8 A secondary objective was to determine if the impact of OHIP+ on the temporal trend in HbA1c differed according to SES. We hypothesized that the temporal trend of HbA1c would improve during OHIP+ and that the change would be greater for those with a lower SES.
Methods
Study design
Using data from administrative data sets, we conducted a population-based trend analysis to assess the impact of OHIP+ on HbA1c among youth younger than 25 years with diabetes in Ontario, from Jan. 1, 2016, to Mar. 31, 2019.
Population and setting
We included all youth who were younger than 21 years, 9 months and residing in Ontario on Jan. 1, 2016, and who were identified to have a diagnosis of diabetes before age 15; we used a population-based diabetes registry derived from administrative data and validated in children.9 We excluded youth with diabetes who were diagnosed on or after Jan. 1, 2015, because they may have been in the early phase of type 1 diabetes, during which there is residual endogenous insulin secretion and better glycemic management. We also excluded those who had their 25th birthday, died or moved out of Ontario before Mar. 31, 2019.
The baseline period was Jan. 1, 2016, to Dec. 31, 2017. The follow-up period was Apr. 1, 2018, to Mar. 31, 2019. We included a washout period from Jan. 1 to Mar. 31, 2018, during which HbA1c values could reflect the period before OHIP+ coverage began.
Data sources
We used the following administrative data sets at ICES in Toronto:10 the pediatric Ontario Diabetes Database, a validated registry of all Ontario residents (aged < 19 yr) with diabetes (83% sensitivity, 99% specificity) to ascertain the cohort and to determine date of diagnosis;9 Ontario Laboratory Information System (OLIS) to ascertain HbA1c (%); Ontario Drug Benefit (ODB) claims (to identify those eligible for social assistance, Trillium drug program claims and OHIP+ claims); the Registered Persons Database (demographics and vital statistics); and the Ontario Marginalization Index (ON-Marg) to assign deprivation quintiles.11 These data sets were linked using unique encoded identifiers and analyzed at ICES.
Outcomes
We extracted baseline characteristics, including age, duration of diabetes, sex and SES, on Jan. 1, 2016. We categorized HbA1c, measured as a percentage, before OHIP+ coverage began as “good,” “moderate” or “poor” if the mean HbA1c for each young person was less than 7.5%, 7.5%–8.9% or 9.0% or greater, respectively, between Jan. 1, 2016, and Apr. 1, 2018. We based our selection of a good HbA1c level as less than 7.5% on the glycemic target in the Diabetes Canada 2018 guideline. We selected the poor HbA1c level as 9.0% or greater because having an HbA1c level of 9.0% or more is associated with an increased risk of diabetic ketoacidosis, a serious acute diabetes complication.12,13
We measured SES using the deprivation dimension of the ON-Marg, which includes indicators of income, education, housing and family structure. Because we used this measure ecologically as a surrogate, we selected this broader metric to capture SES. Material deprivation is an independently validated dimension of the ON-Marg that measures marginalization at the level of the census dissemination area, which represents a population of about 400–700 people, divided into quintiles.11
We extracted all HbA1c values available in OLIS during the study period. It is standard of practice for children with diabetes to have HbA1c measured every 3 months.14 The OLIS contains data from community laboratories and most Ontario hospital laboratories but not from point-of-care HbA1c testing unless it is reported to a hospital laboratory. Some laboratories did not contribute to OLIS during the study period.15 Of those, The Hospital for Sick Children is affiliated with the largest Ontario Pediatric Diabetes Network program and follows about 800 children with diabetes (about 10% of children with diabetes in Ontario).16,17
We extracted any ODB claim before OHIP+ coverage as an indicator of eligibility for social assistance. We extracted all ODB drug claims for insulin and metformin (using the IQVIA Drug Information File to identify the Drug Identification Number for the medications); metformin is often prescribed for adolescents and young adults with type 2 diabetes to estimate the proportion of those likely to have type 2 diabetes. 18 Data were available for publicly paid prescriptions but not for those paid for by private insurance or self-payment.
Our primary outcome was HbA1c level. We extracted all HbA1c values during both time periods. The main exposure was the time period.
Statistical analysis
We described baseline characteristics, including publicly paid drug claims, according to whether individuals made an insulin claim to compare those who were likely to have type 1 diabetes with those who had type 2 diabetes or did not have diabetes. We used all available HbA1c data, and the unit of analysis was the date on which the HbA1c level was measured.
We used segmented regression models to analyze temporal changes in HbA1c and to test our hypotheses about the impact of OHIP+ on temporal trends in HbA1c. Segmented linear regression is a method to analyze temporal data to evaluate the impact of a policy change. We estimated the models using generalized estimating equation methods with an autoregressive covariance structure to account for repeated measurements for individuals.19,20 We excluded young people with missing values for any variable specified in the models from the analyses. We fit an adjusted model without an interaction between deprivation quintiles to test whether temporal trends changed overall between periods and an adjusted model with interaction terms to test whether the change in temporal trends between periods differed across SES strata (Appendix 1, available at www.cmajopen.ca/content/10/2/E519/suppl/DC1). We used SAS version 9.4 M5 to conduct the analyses.
Ethics approval
The use of data for this project is authorized under Section 45 of Ontario’s Personal Health Information Protection Act and does not require review by a research ethics board.
Results
Baseline characteristics for youth aged 21 years, 9 months or younger in Ontario on Jan. 1, 2016, with diabetes diagnosed before age 15 years before Jan. 1, 2015 (n = 9641), according to whether an insulin claim was made are shown in Table 1. Of these youth, 7041 (73.0%) made an insulin claim. We found that youth without insulin claims were slightly younger, had a shorter duration of diabetes and were of slightly lower SES (Table 1). Among those who made an insulin claim, 2466 (35.0%) were eligible for social assistance as indicated by a publicly paid drug claim before OHIP+ coverage began.
Baseline characteristics of youth (aged 21 years, 9 months or younger) who had and did not have an insulin claim during coverage by the Ontario Health Insurance Plan (OHIP)+ program (Jan. 1, 2018, to Mar. 31, 2019)
Of the 2600 individuals who did not make a claim for insulin, 1663 (64.0%) had a claim for a different drug. One hundred sixteen of the 2600 (4.5%) with no insulin claims and 225 of the 7041 (3.2%) with an insulin claim had claims for metformin. Among those in the most deprived quintile who made an insulin claim through OHIP+ (n = 1232), 563 (45.7%) made a publicly paid drug claim before OHIP+ coverage began. There were a similar number of claims for insulin in each quarter during OHIP+ coverage, ranging from 5421 to 5820.
Glycemic management
The number of youths with 1, 2, 3 and 4 or more HbA1c results available were 1062, 869, 657 and 3759, respectively. At least 1 HbA1c result in both study periods was available for 47.2% (4551/9641) of these young people. Among those, 1729 (38.0%), 1906 (41.9%) and 916 (20.1%) had poor, moderate and good baseline glycemic management, respectively. The characteristics of these young people according to HbA1c availability were similar (Appendix 1, Table S1). A higher percentage of those with at least 1 HbA1c result available (4514, 99.2%) had a drug claim compared with 4190/5090 (82.3%) of those who had no available HbA1c result.
Temporal trends in HbA1c
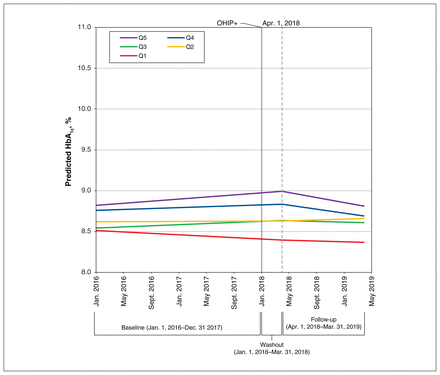
In the analysis with no interactions between deprivation quintiles, we found that the temporal trend in HbA1c was negligibly different between periods (β estimate −0.0002, 95% confidence interval [CI] −0.0004 to 0.0000) (Table 2). In the analysis with interaction terms, the size of the effect of OHIP+ coverage was slightly greater in those in the most-deprived compared with the least-deprived quintile (β estimate −0.0008, 95% CI −0.0015 to −0.0001) (Table 3). Model-based estimates of HbA1c over time (before and after Apr. 1, 2018) by deprivation quintile are shown in Figure 1.
Adjusted segmented regression of hemoglobin A1c before the Ontario Health Insurance Plan (OHIP)+ program began (Jan. 1, 2016, to Dec. 31, 2017) and during OHIP+ (Apr. 1, 2018, to Mar. 31, 2019) to evaluate whether temporal trends changed between time periods (n = 6347 youth; n = 32 802 measurements of hemoglobin A1c)
Adjusted segmented regression of hemoglobin A1c before the Ontario Health Insurance Plan (OHIP)+ program began (Jan. 1, 2016, to Dec. 31, 2017) and during OHIP+ (Apr. 1, 2018, to Mar. 31, 2019) including interaction terms to evaluate if the change in temporal trend of hemoglobin A1c differed according to socioeconomic status (n = 6347 youth; n = 32 802 measurements of hemoglobin A1c)
Predicted hemoglobin A1c (HbA1c, %) over time before and during the Ontario Health Insurance Plan (OHIP)+ program (n = 6347). Linear predictions of HbA1c before OHIP+ (Jan. 1, 2016, to Dec. 31, 2017) and during OHIP+ (Apr. 1, 2018, to Mar. 31, 2019) by deprivation quintile (Q1 = least deprived, Q5 = most deprived).
Interpretation
During 15 months of OHIP+ coverage, 7041 (73.0%) of youth with diabetes made an insulin claim. Among those with an insulin claim, only 2466 (35.0%) made a drug claim before OHIP+ coverage began, which suggests that 4575 (65.0%) became newly eligible for public drug coverage during OHIP+.
Youth with an insulin claim showed a similar SES gradient to previous population-based studies of adolescents and young adults with presumed type 1 diabetes in Ontario.21,22 Those with no insulin claim likely did not have type 1 diabetes, and their SES profile is similar to the overall population of adults with diabetes in Ontario, which has been previously reported.23,24 Contrary to our hypothesis, we did not observe a statistically significant change in the temporal trend of HbA1c results after OHIP+ implementation. The period of 15 months of OHIP+ may not have been enough time to observe a change in the temporal trend in HbA1c.
Our results are similar to the Carefully Selected and Easily Accessible at No Charge Medications (CLEAN Meds) trial, which provided free essential medicines to adult outpatients with type 1 and 2 diabetes who had reported not being able to afford medicines. This study found that although medication adherence improved, there was no change in HbA1c levels.25 The results of the CLEAN Meds trial suggest that pharmacare may help overcome low income as a negative social determinant of health; however, it is insufficient to improve HbA1c levels alone. Policy solutions to address the effects of negative social determinants of health should consider material and social deprivation and health literacy, which are known to be associated with HbA1c.26,27
We found the size of the impact of OHIP+ on HbA1c trajectory was slightly greater for those youth in the most deprived compared with those in the least deprived quintile. Although structured programs to support diabetes self-management are associated with a reduction in HbA1c of 0.5%,28 the Diabetes Control and Complications Trial showed that proportional reductions in HbA1c are associated with proportional reductions in the risk of diabetes complications.29 On an individual level, a change in HbA1c of 0.5% is clinically important;29 at a population level, even a small improvement is meaningful. Two population-based studies involving adults with diabetes in Ontario found reduced disparity in diabetes outcomes between the lowest and highest income groups in people older than 65 years of age who had publicly funded prescription drug coverage than in those younger than 65 years.23,24 However, these studies primarily included people with type 2 diabetes, for whom medications are arguably discretionary.
Cost-related nonadherence for any prescription medication was reported on the Canadian Community Health Survey in 9.4% of people 12–34 years of age in 200730 and in 2.06% of those aged 12–17 years in 2016.31 Furthermore, youth 12–18 years of age were more likely to spend less on other areas because of drug costs, which suggests that parents prioritized spending on prescription medicines for children.30 This is supported by our finding that the number of insulin claims during the OHIP+ period was constant. However, youth in the lowest SES, who must purchase insulin whether or not they have private insurance, may have slightly improved glycemic management because they may have been rationing insulin previously or may have less financial stress and are able to spend on other necessities such as healthy food, which, in turn, may be associated with improved glycemic management.
Among youth in the most deprived quintile who made an insulin claim, only 563 (45.7%) made any public drug claim before OHIP+. This suggests that for those in the lowest SES, OHIP+ alleviated the financial burden associated with the cost of insulin. Cost is known to be a barrier to accessing medications in Canada.32 In addition, user fees, copayments or limits on the quantity of subsidized prescriptions create barriers to essential medications and are associated with worse health outcomes in people whose medication use is most likely to be affected by these cost-sharing measures.33
Beginning Apr. 1, 2019, eligibility criteria for OHIP+ changed to exclude Ontario youth younger than 25 years who had any private drug coverage, regardless of its comprehensiveness or the extent of the copayment required. Therefore, many youth who are younger than 25 years may have financial barriers to insulin. For youth with low incomes, even small financial barriers, such as low prescription fees or copayments, have substantial effects on access to medication and by reducing expenditures on other essential items for health.34,35 In Canada, households with the lowest SES have the highest proportion of out-of-pocket expenses on drugs.36 This suggests that children from the most deprived households who have type 1 diabetes are more likely to be affected negatively by costs of life-sustaining medications.
Limitations
We expected close to 100% of youth to make an insulin claim because insulin is life sustaining for those with type 1 diabetes;12 it is unlikely that people would go more than 15 months without filling a prescription; and OHIP+ was the first payer for medications.7 It is likely that some youth did not have type 1 diabetes: some of these youth may have had non–type 1 diabetes or were identified erroneously as having diabetes.
There is a potential for selection bias owing to missing HbA1c data in OLIS from The Hospital for Sick Children and other smaller hospital laboratories; however, there are no clinically important differences in the characteristics of those patients with and without HbA1c data. There was a relatively high proportion of youth with no HbA1c data who did not make a drug claim. It is possible that these youth either did not have diabetes or had HbA1c tests done at laboratories that did not contribute data to OLIS. Slightly more young people with no HbA1c results were in the most deprived quintile. This may reflect that people with lower SES receive fewer tests for chronic diseases.37 Data about race and ethnicity were unavailable; these are known to be associated with HbA1c.38
We were unable to distinguish between diabetes types; however, most children younger than 15 years with a diagnosis of diabetes in Ontario had type 1.39,40 That only 3.5% of individuals in the cohort made a drug claim for metformin, a mainstay in the treatment of pediatric type 2 diabetes,18 further supports this assumption.
Assessment of other important measures of health and well-being is needed to determine the full effect of OHIP+. A 2019 qualitative concept mapping study in Canada reported that those with access to free medicine had reduced stress, better communication with physicians, improved quality of life and a decreased need to sacrifice other essential items for health and well-being.41 It is possible that OHIP+ had a positive effect on other important patient-reported outcome measures such as diabetes distress, diabetes-related quality of life and those described in this mapping study.
Conclusion
We found that a large proportion of youth did not access insulin despite publicly funded pharmacare. This may reflect limitations in our ability to identify those with type 1 diabetes using the available data. The impact of publicly funded pharmacare on HbA1c was slightly greater for youth of lowest SES than for those of highest SES.
Publicly funded pharmacare may be an effective policy tool to combat worsening socioeconomic disparities in diabetes care and outcomes by alleviating the financial burden and improving other broadly defined health and quality-of-life outcomes for children and youth who require life-sustaining medications. The effects of gaps in coverage created by recent changes to OHIP+ eligibility should be evaluated in future analysis of data beyond March 2019.
Acknowledgement
The authors thank IQVIA Solutions Canada Inc. for use of their Drug Information File.
Footnotes
Competing interests: Rayzel Shulman has received speaker fees from Dexcom. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Rayzel Shulman and Baiju Shah conceived the study and developed the study protocol. Rayzel Shulman, Baiju Shah, Karl Everett and Peter Austin contributed to the study protocol. Karl Everett carried out the data analysis. Mohana Giruparajah drafted the initial manuscript. All of the authors contributed to the interpretation of the analysis, reviewed and revised the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was supported by a Physician Services Incorporated New Investigator grant and by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-Term Care.
Data sharing: The data set from this study is held securely in coded form at ICES. While legal data-sharing agreements between ICES and data providers (e.g., health care organizations and government) prohibit ICES from making the data set publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at https://www.ices.on.ca/DAS (email: das{at}ices.on.ca). The full data set creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
Disclaimer: This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH). The opinions, results and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOH is intended or should be inferred.
Parts of this study are based on data and information compiled and provided by the Ontario MOH and the Canadian Institute for Health Information. The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/10/2/E519/suppl/DC1.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/
References
- © 2022 CMA Impact Inc. or its licensors