Abstract
Background: Geographic trends in antibiotic prescribing show regional variation in antibiotic overuse and antimicrobial resistance, posing a threat to global health care systems. This study’s objective was to examine interprovincial variation in outpatient antibiotic dispensing in Canada in 2019.
Methods: We conducted a cross-sectional study of antibiotic prescriptions dispensed in Canadian provinces in 2019, leveraging the IQVIA Geographic Prescription Monitor database. We report annual rates of overall antibiotic dispensing, broad-spectrum antibiotic dispensing and age-specific antibiotic dispensing as prescriptions per 1000 population in each province and nationally.
Results: A total of 23 406 640 antibiotic prescriptions were dispensed nationally in 2019, at a rate of 627.3 prescriptions per 1000 population. Overall antibiotic dispensing rates in Newfoundland and Labrador (920.5 prescriptions per 1000 population) and Saskatchewan (713.7 prescriptions per 1000 population) significantly exceeded the national rate, whereas the rate in British Columbia (543.3 prescriptions per 1000 population) was significantly below the national rate. We observed additional variation when provincial rates of antibiotic dispensing were stratified by drug class and age group.
Interpretation: We identified interprovincial variation in antibiotic use in Canadian provinces in 2019. These findings highlight the need for provincial targets for antibiotic use to reduce overuse and antimicrobial resistance.
Antimicrobial resistance is a recognized threat to human health both in Canada and internationally.1,2 A recent report estimates that antimicrobial resistance was directly responsible for 5400 deaths and $1.4 billion in health care costs in Canada in 2018.3 Antibiotic use is considered the most important driver of antimicrobial resistance. 4,5 An American study estimates that up to 50% of antibiotic prescribing is inappropriate or unnecessary, suggesting that the risk posed by antibiotic use is modifiable.6 Another study from the United States has identified the relation between geographic variability in outpatient antibiotic use and resistance rates among invasive pneumococcal disease isolates.7 Although reports from the early 2000s suggest declines in antibiotic use in Canada, more recent data suggest that annual antibiotic consumption in Canada has been rising since 2014.8,9 The success of interventions to combat antimicrobial resistance requires a comprehensive understanding of antibiotic use to identify problematic patterns of use and inform targeted interventions.1
In Canada, systematic efforts to control antimicrobial resistance began in 1997.10 Antimicrobial stewardship programs have now been present in both community hospitals and large teaching centres in Canada for more than a decade.11,12 Although these programs have reported benefits at the institutional level, about 90% of antibiotic use in Canada occurs in the community setting, where no such requirements exist and effective antimicrobial stewardship interventions are considerably more challenging to implement.9 High-priority opportunities for improved antibiotic prescribing in this setting include conditions for which antibiotics are overprescribed and conditions for which broad-spectrum antibiotics are overprescribed.13 Therefore, surveillance of antibiotic use in the community should pertain to both overall antibiotic use and the use of specific high-risk agents.
Since Canadian health care is organized and delivered at the provincial level, community antimicrobial stewardship programs are frequently implemented at that same level.14–16 Interprovincial variation in antibiotic use thus becomes a valuable indicator of the degree of unnecessary antibiotic prescribing, which is essential to inform national targets for reduction of antibiotic use. The Public Health Agency of Canada regularly publishes data on outpatient antimicrobial use in Canada.9 Although previous publications have used statistical approaches to assess the significance of interprovincial variation in antimicrobial use, the most recent editions of these reports use 2010 data.17,18
Given the ever-changing landscape of antimicrobial use and resistance, the objective of this study was to examine interprovincial variation in antibiotic prescribing in the community setting in Canada in 2019, comparing overall antibiotic dispensing, broad-spectrum antibiotic dispensing and age-specific antibiotic dispensing across provinces.
Methods
Study design and setting
We conducted a cross-sectional analysis of antibiotic prescriptions in Canadian provinces from Jan. 1 to Dec. 31, 2019. This analysis captured prescriptions from all 10 Canadian provinces and no Canadian territories (data were not available from the territories).
Data sources
Prescription data were obtained from the IQVIA Geographic Prescription Monitor database, which uses transactional data from a panel of about 6100 retail pharmacies. At the national level, more than 75% of total prescriptions dispensed are accounted for in the database (based on June 2020 data) (Ms. Josiane Gaudet, IQVIA, Montréal, Que.: personal communication, 2021). The database uses a patented geospatial methodology to estimate total prescriptions dispensed at a geographic level. The Geographic Prescription Monitor database also leverages other IQVIA data assets (claims and distribution) as input into the projection methodology. The survey design yielded market-level yearly national estimates that have a sampling error (standard error) of 6% for antibiotics (prescriptions per 1000 population). At the provincial level, the sampling error can reach slightly higher levels, although it will not exceed 12%. While the IQVIA methodology is proprietary, its data are used regularly for research purposes and have been externally validated for some specific measures of antibiotic prescribing and use.19–23
We included data from the IQVIA database on oral antibiotics, which were sorted by drug class into 14 groups: first-generation cephalosporins, second- and third-generation cephalosporins, second-generation fluoroquinolones, third-generation fluoroquinolones, lincosamides, macrolides, metronidazole, nitrofurantoin, narrow-spectrum penicillins, penicillins with β-lactamase inhibitors, tetracyclines, trimethoprim and/or sulfonamides, vancomycin and other antibiotics (Appendix 1, available at www.cmajopen.ca/content/10/1/E262/suppl/DC1). Antitubercular agents were excluded from the analysis.
Annual population estimates were obtained from Statistics Canada by province and age group in 2019.24 For age group analyses, people younger than 18 years were defined as children, those aged 18–64 years were defined as adults and those older than 64 years were defined as older adults.
Statistical analysis
We calculated the rate of oral antibiotic prescription dispensing per 1000 population in 2019 by province and nationally for all included antibiotics. We evaluated overall dispensing rates, as well as dispensing rates for specific broad-spectrum antibiotics: macrolides, fluoroquinolones and penicillins with β-lactamase inhibitors. Additionally, we calculated provincial and national dispensing rates stratified by age group. Age-adjusted rates were calculated using the direct standardization method. These age adjustments used Canada as a referent and were based on the proportion of 7 age groupings (< 6 yr, 6–13 yr, 14–18 yr, 19–29 yr, 30–49 yr, 50–64 yr and > 65 yr) in each provincial population.
We built Poisson models to test the hypothesis that variability existed between the provinces for both crude and age-adjusted antibiotic dispensing rates. The number of prescriptions was used as the dependent variable, and population, in thousands, was used as the offset. We used type III tests (F statistics) to assess the overall significance of that variability. We defined significance as p < 0.05. Microsoft Excel was used for data cleaning and analysis, and SAS was used for the statistical analysis.
Ethics approval
This study used anonymized aggregate data and did not require research ethics board approval.
Results
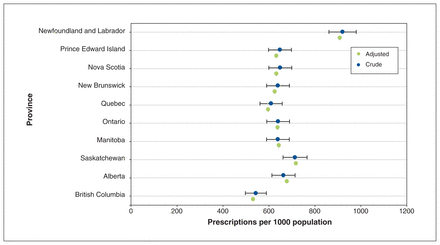
Across Canada, a total of 23 406 640 antibiotic prescriptions were filled at outpatient pharmacies in 2019, at a rate of 627.3 prescriptions per 1000 population (Figure 1). Crude dispensing rates varied between provinces, from 543.3 prescriptions per 1000 population in British Columbia to 920.5 prescriptions per 1000 population in Newfoundland and Labrador. The crude antibiotic dispensing rate in Saskatchewan was 713.7 prescriptions per 1000 population. The type III test for overall variation yielded p < 0.001, indicating significant interprovincial variation. Full model results are available in Appendix 2 (www.cmajopen.ca/content/10/1/E262/suppl/DC1).
Crude and age-adjusted antibiotic prescription rates per 1000 population with 95% confidence intervals. Poisson model outputs are available in Appendix 2 (www.cmajopen.ca/content/10/1/E262/suppl/DC1).
Age-adjusted dispensing rates ranged from 531.5 prescriptions per 1000 population in BC to 908.8 prescriptions per 1000 population in Newfoundland and Labrador. The type III test for overall variation between age-adjusted provincial dispensing rates also yielded p < 0.001. Narrow-spectrum penicillins (28.6%), macrolides (14.0%), first-generation cephalosporins (9.1%), tetracyclines (7.6%) and penicillins with β-lactamase inhibitors (7.6%) were the 5 most commonly prescribed antibiotic classes nationally in 2019 (Appendix 3, available at www.cmajopen.ca/content/10/1/E262/suppl/DC1).
Broad-spectrum antibiotics
In 2019, the national dispensing rate of fluoroquinolones was 56.8 prescriptions per 1000 population, the rate of macrolides was 88.0 and the rate of penicillins with β-lactamase inhibitors was 47.6 (Figure 2). The type III tests for overall variation in both fluoroquinolone and macrolide dispensing rates yielded p < 0.001, indicating significant interprovincial variation. For penicillins with β-lactamase inhibitors, the test yielded p = 0.3. Full model results are available in Appendix 2.
Rate of outpatient prescription of broad-spectrum oral antibiotics per 1000 population in Canada in 2019, stratified by drug class and province. Poisson model outputs are available in Appendix 2 (www.cmajopen.ca/content/10/1/E262/suppl/DC1).
Provincial rates of macrolide dispensing ranged from 58.1 prescriptions per 1000 population in BC to 125.7 prescriptions per 1000 population in Newfoundland and Labrador. Provincial rates of penicillin with β-lactamase inhibitor dispensing ranged from 41.0 prescriptions per 1000 population in BC to 70.3 prescriptions per 1000 population in Newfoundland and Labrador. Provincial rates of fluoroquinolone dispensing ranged from 40.0 prescriptions per 1000 population in Prince Edward Island to 86.4 prescriptions per 1000 population in Newfoundland and Labrador. Nationally, fluoroquinolones represented 9.1% of total antibiotic prescriptions. Provincially, fluoroquinolones ranged from 6.2% of total antibiotic prescriptions in Prince Edward Island to 13.5% of total antibiotic prescriptions in Quebec. Trends in second-generation fluoroquinolone dispensing did not differ from trends in total fluoroquinolone dispensing (Figure 2; Appendix 4, available at www.cmajopen.ca/content/10/1/E262/suppl/DC1). Third-generation fluoroquinolone dispensing was highest in Ontario and Quebec (Appendix 4).
Age-specific trends in antibiotic dispensing
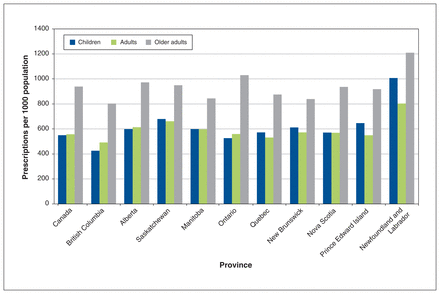
The national rate of antibiotic dispensing for children was 549.8 prescriptions per 1000 population, the rate for adults was 557.2 and the rate for older adults was 938.9 (Figure 3; Appendix 5, available at www.cmajopen.ca/content/10/1/E262/suppl/DC1). The type III test for overall variation in dispensing rates within all 3 age groups yielded p < 0.001. Full model results are available in Appendix 2.
Rate of outpatient prescription of antibiotics per 1000 population in Canada in 2019, stratified by patient age group and province. Poisson model outputs are available in Appendix 2 (www.cmajopen.ca/content/10/1/E262/suppl/DC1).
Newfoundland and Labrador had the highest rates of antibiotic dispensing for all age groups, with rates of 1007.1 prescriptions per 1000 population among children, 803.6 among adults and 1210.5 among older adults (Figure 3). British Columbia had the lowest rates of antibiotic dispensing for all age groups, with rates of 425.8 prescriptions per 1000 population among children, 492.3 among adults and 803.2 among older adults (Figure 3).
Interpretation
In this cross-sectional study, we reported an annual dispensing rate for oral antibiotics filled at Canadian community pharmacies of 627 prescriptions per 1000 population in 2019. We observed variation in the patterns of antibiotic dispensing across Canadian provinces, and we noted interprovincial variation in dispensing for broad-spectrum antibiotic classes and within specific age groups. Our findings align with those of a previous study showing relatively high rates of antibiotic dispensing in Newfoundland and Labrador and relatively low dispensing rates in BC.9 The variation observed in the current study has relevance to the development of national and provincial targets for reduction of antibiotic use in Canada.
The observed national annual dispensing rate in our study was 5.8% lower than the rate observed in 2009, despite an increase in the average age of the Canadian population. 25 It is below the dispensing rate recently observed in the US (791 per 1000 population), but substantially higher than European prescribing rates that were recently observed (563 in England, 450 in Norway and 285 per 1000 population in Sweden).26–29
Both international and interprovincial variation in prescribing rates may be in part due to heterogeneity in stewardship initiatives. In BC, where antibiotic use is consistently lower than in other Canadian provinces, provincially funded stewardship programs, in place since 2005, have led to a substantial reduction in antibiotic use.30 Similarly, through the formal dedication of resources at the local and national level since 1995, Sweden has substantially reduced national antibiotic use and antimicrobial resistance to some of the lowest levels in the world.31 These findings highlight the importance of sustained dedication of resources to antimicrobial stewardship programs for long-term success, and indicate substantial overprescribing of antibiotics in most parts of the world. The differences in scope and timing of interventions are important to consider when analyzing interprovincial variation in rates of antibiotic prescribing.
Given that antibiotic use is the largest modifiable driver of antimicrobial resistance, there is an urgent need to reduce use in Canada and globally. The US, for example, was working toward the goal of reducing inappropriate antibiotic prescribing by 50% by 2020.32 Owing in part to the challenges associated with assessing prescription appropriateness at the population level, many European nations have targeted reductions in overall human antibiotic use of 15%–50%.28,33,34 The Chief Public Health Officer of Canada recently identified the need for detailed research on antibiotic use and appropriateness to explain interprovincial variation and to develop appropriate Canadian targets for antibiotic use.35 A study of primary care practices in Ontario identified that 15% of visits for an infectious indication were associated with unnecessary antibiotic prescribing and that about 25% of all antibiotic prescriptions were likely unnecessary, suggesting an opportunity for safely reducing antibiotic prescribing in Canadian primary care.36
Targets for antibiotic use in Canada should also be developed for the prescribing of specific high-risk drug classes. We observed that although the overall dispensing rate in Quebec was similar to the national rate, the provincial rate of fluoroquinolone dispensing was significantly higher. In Belgium, a country with historically high antibiotic use, the national target of a 50% reduction in total antibiotic consumption was accompanied by a targeted fourfold reduction in fluoroquinolone consumption to 5% of total antibiotic consumption.34 In our study, fluoroquinolones represented 9.1% of total antibiotic consumption nationally and 13.5% of total antibiotic consumption in Quebec. In Prince Edward Island, where the fluoroquinolone dispensing rate was the lowest in Canada, they still exceeded the Belgian target, at 6.2% of total antibiotic consumption. Compared with broad-spectrum agents, narrow-spectrum agents are favourable because they are less likely to select for resistance in non–target bacterial species or multidrug resistance.37 Apart from resistance, fluoroquinolones also carry risk of Clostridioides difficile–associated diarrhea, tendinopathy, peripheral neuropathy and central nervous system disorders.38,39 Although rates of fluoroquinolone dispensing in Canada have declined since 2000, they still exceed international targets.18 Therefore, when seeking to improve antibiotic use in the community setting, we should consider the reduction of overall prescribing and prescribing of high-risk and broad-spectrum antibiotics.
As medical needs vary across populations, it would be prudent to consider patient factors when setting targets for antibiotic use. Multiple studies have shown that interphysician variability in antibiotic prescribing cannot be explained by patient factors alone.40,41 Given that a substantial portion of variation occurs at the physician level, peer-comparison feedback interventions for high-prescribing physicians are an important component of community antimicrobial stewardship programs.13 In some jurisdictions, pay-for-performance targets have been developed to encourage primary care physicians to meet objectives.42,43 Although formulary restrictions offer another tool to guide prescribing from an antimicrobial stewardship perspective, they have not been found to influence outpatient antibiotic dispensing in the Canadian context.44
Limitations
Several limitations of our study design warrant discussion. Although Statistics Canada classifies children as 0–17 years of age, IQVIA includes those aged 18 years in this age group. Therefore, the age groups did not perfectly overlap between data sources, although the large age groupings were still able to capture trends in dispensing. Second, the IQVIA Geographic Prescription Monitor database does not provide patient-level data. Therefore, we were unable to assess the appropriateness of prescriptions, as this data source does not capture individual-level information, such as patient diagnoses. We were also unable to identify situations in which a single patient received multiple antibiotics at once or within a given year. Prescriptions at hospital-operated long-term care facilities are likely not captured in our data set, as they would typically be dispensed through hospital pharmacies, contributing a potential confounding factor to our results as provinces may leverage differing mechanisms for dispensing drugs.
Although the provincial-level sampling error of the data source rarely exceeds 5% to 10%, recent estimates suggest that prescription projections in small jurisdictions may be subject to additional variability.45 We were unable to assess goodness-of-fit in our Poisson models, so possible overdispersion in the data could not be accounted for statistically. Lastly, although we compared the initiation and selection of antibiotics, this study did not assess duration of therapy, which is an indicator of inappropriate antimicrobial use.
Conclusion
We observed substantial variation in the rate of outpatient antibiotic dispensing across Canadian provinces and age groups. We observed particularly high rates of overall antibiotic dispensing in Newfoundland and Labrador and Saskatchewan. These data may inform minimum benchmarks or goals for provincial targets for antibiotic use, as, when compared internationally, it is likely that substantial overprescribing is occurring in all Canadian provinces. These data may also be used as a benchmark for future analyses of antibiotic use in Canadian provinces, and to inform prioritization of targeted stewardship projects.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: All of the authors were involved in the study conception and design, data analysis and interpretation, and drafting the manuscript and revising it for important intellectual content. All authors approved the final version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was funded by a grant from the Ontario Ministry of Health. The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources.
Data sharing: This study was an analysis of a claims database using data obtained under licence from IQVIA Canada Inc. The raw data cannot be publicly shared as it was obtained from a third party and as per signed agreement. Requests for data can be sent to IQVIA Solutions Canada Inc. and may carry a cost.
Disclaimer: The statements, findings, conclusions, views and opinions contained and expressed in the report are based in part on data obtained under licence from IQVIA Solutions Canada Inc. concerning the following information service(s): Geographic Prescription Monitor database, data period Jan. 1, 2019, to Dec. 31, 2019. All rights reserved. The statements, findings, conclusions, views and opinions expressed herein are not necessarily those of IQVIA Inc. or any of its affiliated or subsidiary entities.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/10/1/E262/suppl/DC1.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/
References
- © 2022 CMA Impact Inc. or its licensors