Article Text
Abstract
Background Among patients who had an ischaemic stroke presenting directly to a stroke centre where endovascular thrombectomy (EVT) is immediately available, there is uncertainty regarding the role of intravenous thrombolysis agents before or concurrently with EVT. To support a rapid guideline, we conducted a systematic review and meta-analysis to examine the impact of EVT alone versus EVT with intravenous alteplase in patients who had an acute ischaemic stroke due to large vessel occlusion.
Methods In November 2021, we searched MEDLINE, Embase, PubMed, Cochrane, Web of Science, clincialtrials.gov and the ISRCTN registry for randomised controlled trials (RCTs) comparing EVT alone versus EVT with alteplase for acute ischaemic stroke. We conducted meta-analyses using fixed effects models and assessed the certainty of evidence using the GRADE approach.
Results In total 6 RCTs including 2334 participants were eligible. Low certainty evidence suggests that, compared with EVT and alteplase, there is possibly a small decrease in the proportion of patients independent with EVT alone (risk ratio (RR) 0.97, 95% CI 0.89 to 1.05; risk difference (RD) −1.5%; 95% CI −5.4% to 2.5%), and possibly a small increase in mortality with EVT alone (RR 1.07, 95% CI 0.88 to 1.29; RD 1.2%, 95% CI −2.0% to 4.9%) . Moderate certainty evidence suggests that there is probably a small decrease in symptomatic intracranial haemorrhage (sICH) with EVT alone (RR 0.75, 95% CI 0.52 to 1.07; RD −1.0%; 95%CI −1.8% to 0.27%).
Conclusions Low certainty evidence suggests that there is possibly a small decrease in the proportion of patients that achieve functional independence and a small increase in mortality with EVT alone. Moderate certainty evidence suggests that there is probably a small decrease in sICH with EVT alone. The accompanying guideline provides contextualised guidance based on this body of evidence.
PROSPERO registration number CRD42021249873.
- stroke
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
When possible, acute ischaemic stroke due to large vessel occlusion is managed with endovascular thrombectomy (EVT) and intravenous alteplase; however, whether combination therapy is superior to EVT alone is uncertain.
WHAT THIS STUDY ADDS
Low certainty evidence (rated down due to very serious imprecision) from six randomized trials suggests that treatment of acute stroke due to large vessel occlusion with EVT alone, versus EVT with alteplase, may slightly decrease the proportion of patients that achieve functional independence and slightly increase mortality. Moderate certainty evidence shows that EVT alone probably results in a small decrease in symptomatic intracranial haemorrhage.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
Further trials are required to establish whether combination therapy is superior to EVT alone for acute stroke due to large vessel occlusion, and EVT alone is probably associated with a lower risk of harms. Clinical practice guidelines should consider these findings to optimise evidence-based care of acute stroke.
Introduction
Over 2.7 million people die of ischaemic stroke each year, and many who recover are left with permanent disabilities.1 Approximately 21% of acute ischaemic stroke are due to large vessel occlusion2 for which the standard of care has historically been intravenous alteplase, a thrombolytic medication.3 More recently, direct mechanical reperfusion with endovascular thrombectomy (EVT) has proven effective.4 Both treatments are extremely time-sensitive, and delays of 15 min in treatment initiation are associated with worse outcomes.
Among patients who had an ischaemic stroke are eligible for and can be treated with both interventions immediately, there has been uncertainty regarding the role of intravenous alteplase.5 6 Thrombolytic agents, such as alteplase, may contribute to early reperfusion of the ischaemic area and resolve residual distal thrombi after EVT.7–11 For large, proximally located thrombi, however, the rate of early recanalisation is low in the first hour following alteplase administration, and fragmentation with distal embolisation of the target thrombus can result in worsening distal perfusion, potentially complicating EVT.5 12
In the last 18 months, six randomized trials have been completed that provide evidence to address this uncertainty.13–18 We conducted a systematic review and meta-analysis to explore the benefits and harms of EVT with or without intravenous alteplase for acute ischaemic stroke due to large vessel occlusion. Our findings supported the development of a clinical practice guideline (Personal communication: Ye Z, Busse J, Hill M. Endovascular thrombectomy and intravenous alteplase in patients with acute ischemic stroke: a rapid clinical practice guideline. 2022).
Methods
We followed the Preferred Reporting Items for Systematic Review and Meta-Analysis checklist19 when writing our report. All subjective decisions (ie, study selection, data abstraction, risk-of-bias assessment) were made in duplicate by independent reviewers, and any disagreements were resolved by discussion or by referral to a third reviewer.
Guideline panel involvement
A guideline panel provided critical oversight of different steps of this review, including: (1) defining the study question; (2) prioritising outcome measures; and (3) informing if measures of precision associated with pooled effect estimates were imprecise. The panel included seven general stroke experts, three neurointerventionalists, six methodologists, four patient partners who had recovered from an acute ischaemic stroke and received thrombectomy with or without intravenous thrombolysis, one caregiver, two academic pharmacists, one emergency physician and one health economist. All patients received personal training and support to optimise contributions throughout the guideline development process. The members of the guideline panel led the interpretation of the results based on what they expected the typical values and preferences of patients to be, as well as the variation between patients.
Data sources and search strategy
We searched MEDLINE, Embase, PubMed, Cochrane Central Register of Controlled Trials, Web of Science, clincialtrials.gov and the International Standard Randomized Controlled Trial Number (ISRCTN) registry from inception to 22 November 2021. No language restrictions were applied, and a research information specialist (RJC) developed all database-specific search strategies (online supplemental appendix 1). We reviewed the reference lists of all included studies and relevant systematic reviews for additional eligible trials. In addition, we searched abstracts for the past 3 years of proceedings of the International Stroke Conference, European Stroke Conference, Asia-Pacific Stroke Meeting and the World Stroke Congress.
Supplemental material
Study selection
We included randomized controlled trial (RCTs) that enrolled patients who had an acute ischaemic stroke due to large vessel occlusion and randomised them to receive EVT with intravenous alteplase versus EVT alone. Pairs of reviewers independently screened titles and abstracts and reviewed the full texts of potentially eligible studies.
Data extraction
Each eligible trial underwent duplicate data abstraction by pairs of reviewers working independently, who collected study characteristics, patient information including number enrolled, age, sex, comorbidities, stroke mechanism and clot location of participants, treatment details, and all patient-important outcomes: recovery with minimal disability (modified Rankin Scale (mRS) Score of 0–2), symptomatic intracranial haemorrhage (sICH), mortality and procedure-related complications.
Risk-of-bias assessment
Using a modified Cochrane risk-of-bias instrument, pairs of reviewers independently assessed each article for risk of bias considering sequence generation, allocation sequence concealment, blinding of participants, healthcare providers, data collectors, outcome assessor/adjudicator and missing outcome data (≥10% missing data were considered high risk of bias).20 Response options for each item were ‘definitely or probably yes’ (assigned a low risk of bias) and ‘definitely or probably no’ (assigned a high risk of bias).21
Data analysis
We conducted fixed effects meta-analysis using the Mantel-Haenszel method to calculate risk ratios (RRs) and risk differences (RDs), and the associated 95% CI, for all patient-important outcomes reported by more than one study. For computing RDs and 95% CIs, we applied the RRs to the baseline risks from a high-quality observational study of 6350 ischaemic stroke from 42 centres that received EVT with or without intravenous alteplase.22 We conducted a post-hoc sensitivity analysis excluding the SKIP trial14 from our analyses on the basis that the dose of alteplase may affect results. Specifically, the SKIP trial administered alteplase at a dose of 0.6 mg/kg vs 0.9 mg/kg in other trials.
We performed all statistical analyses using Review Manager for Windows (RevMan, V.5.3). Comparisons were two-tailed using a p≤0.05 threshold.
Assessment of certainty of evidence
The authors and the guideline panel achieved consensus in categorising the certainty of evidence for all reported outcomes as high, moderate, low or very low using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.23 With the GRADE approach, RCTs start as high certainty evidence,23 but may be rated down for risk of bias,24 imprecision,25 indirectness,26 inconsistency27 or publication bias.28 We also rated down significant effects for imprecision if they were informed by <300 patients for continuous outcomes or <300 events for dichotomised outcomes.25 We did not rate down for risk of bias if the only criterion not met was blinding of study participants or personnel on the basis that a recent meta-epidemiological study found no evidence for an average difference in estimated treatment effect between trials with and without blinded patients, healthcare providers or outcome assessors.29 We also did not rate down the same effect estimate two times for both inconsistency and imprecision.
Rating of imprecision was fully contextualised by the guideline panel,30 and we followed GRADE guidance for communicating our findings.31 We presented our evidence syntheses in a GRADE summary of findings tables as both relative and absolute effects to optimise interpretability. The minimally important difference (MID) was informed by a survey of guideline panel members’ views of patient values and preferences, and their subsequent discussion. The thresholds for MID were 1% for recovery with minimal disability, 0.8% for mortality and 1% for sICH; the panel, however, acknowledged both their uncertainty around patient values and likely large variability between patients. We assessed inconsistency among studies by differences in point estimates and overlap of the CI, and the I2 statistic. According to Cochrane Review Handbook, an I2 of 0%–40% might not be important, 30%–60% may represent moderate heterogeneity, 50%–90% may represent substantial heterogeneity and 75%–100% indicates considerable heterogeneity.32
Results
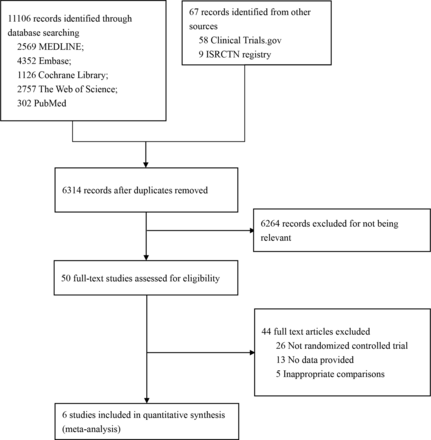
Of 11 121 citations, 4 published RCTs13–16 including 1633 patients and 2 RCTs described at conference presentations17 18 including 701 patients met eligibility criteria (figure 1). Characteristics of included clinical trials, which were all published in 2020 and 2021, are presented in online supplemental appendix 2. Sample size ranges from 200 to 700 and two doses of alteplase (0.6 mg/kg14 and 0.9 mg/kg,13 ,15–18) were administered to participants. All eligible trials adequately generated their randomisation sequence, appropriately concealed allocation, blinded outcome assessors and reported <10% missing outcome data. Due to the nature of the interventions, patients and healthcare providers were unblinded (online supplemental appendix 3).
Flow chart for study selection.
Outcomes for EVT with intravenous alteplase versus EVT alone
Recovery with minimal disability (mRS Score 0–2)
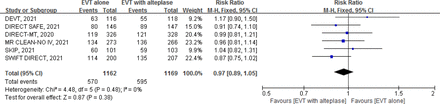
Low certainty evidence from 6 RCTs13–18 (2331 patients) suggests that, compared with EVT with alteplase, EVT alone possibly results in a small decrease in the proportion of patients that achieve functional independence (RR 0.97, 95% CI 0.89 to 1.05; RD −1.5%; 95% CI −5.4% to 2.5%) (figure 2, table 1).
Forest plot for endovascular thrombectomy (EVT) alone versus EVT with intravenous alteplase for modified Rankin Scale (mRS) score 0–2.
GRADE summary of findings for EVT alone versus EVT with alteplase in patients who had an acute ischaemic stroke secondary to large vessel occlusion
Mortality
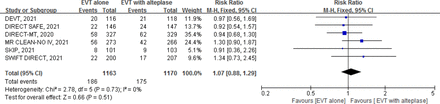
Low certainty evidence from 6 RCTs13–18 (2333 patients) suggests that, compared with EVT with alteplase, EVT alone possibly results in a small increase in mortality (RR 1.07, 95% CI 0.88 to 1.29; RD 1.2%, 95% CI −2.0% to 4.9%) (figure 3, table 1).
Forest plot for endovascular thrombectomy (EVT) alone versus EVT with intravenous alteplase for mortality.
Symptomatic intracranial haemorrhage (sICH)
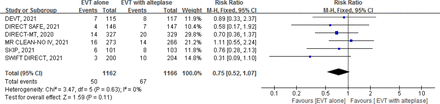
Moderate certainty evidence from 6 RCTs13–18 (2328 patients) suggests that, compared with EVT with alteplase, EVT alone probably results in a small decrease in sICH (RR 0.75, 95% CI 0.52 to 1.07; RD −1.0%; 95% CI −1.8% to 0.27%) (figure 4, table 1).
Forest plot for endovascular thrombectomy (EVT) alone versus EVT with intravenous alteplase for symptomatic intracranial haemorrhage.
Sensitivity analysis excluding the SKIP trial14 did not appreciably change recovery with minimal disability (mRS Score 0–2), mortality and sICH (online supplemental appendix 4).
Procedure-related complications
Overall, 2 studies13 15 including 886 patients reported on procedure-related complications and the results showed no significant difference in procedure-related complications for EVT with or without alteplase (RR 0.89, 95% CI 0.69 to 1.15, p=0.38; online supplemental appendices 5 and 6).
Interpretation
For patients who had an ischaemic stroke with large vessel occlusion who present to comprehensive stroke centres and are eligible for both immediate thrombolysis and EVT, compared with EVT and intravenous alteplase, low certainty evidence suggests that there is possibly a small decrease in the proportion of patients that achieve functional independence and a small increase in mortality with EVT alone; CI are wide with very serious imprecision. Moderate certainty evidence suggests that there is probably a small decrease in sICH with EVT alone. Considering the small differences with very serious imprecision, this evidence supports only weak recommendations for future clinical care. The accompanying guideline33 provides contextualised guidance based on this body of evidence.
Strengths of our systematic review include a comprehensive search for eligible RCTs in any language, and independent study selection, data abstraction and the risk-of-bias assessment by paired reviewers. We engaged a guideline panel of patients and clinical experts to fully contextualise our assessment of the evidence, and to establish MIDs for all outcomes. We used the GRADE approach to assess the certainty of evidence and converted all pooled relative effects to RDs to facilitate interpretation.
Compared with two recent published systematic reviews addressing EVT alone versus EVT with intravenous thrombolysis in acute ischaemic stroke from large vessel occlusion,34 35 our review had the following distinctions. First, we used the GRADE approach to evaluate the certainty of evidence, which formally acknowledges imprecision in effect estimates. The results of our study suggested that EVT alone may decrease the proportion of patients that achieve functional independence and increase mortality, whereas previous systematic reviews concluded no difference between groups in functional independence and mortality. Second, we engaged a guideline panel, which involved patient partners, to contextualise the findings—including assessment of precision associated with pooled effect estimates. Third, prior reviews reported both patient-important and surrogate outcomes. In the systematic review of four trials, surrogate endpoints (successful reperfusion and any intracranial haemorrhage) showed significant improvement, the first favouring EVT plus alteplase and the second favouring EVT alone, and the authors did not address this issue.34 In the systematic review of three trials, there were no significant differences in successful reperfusion.35 Surrogate outcomes are less important when we have evidence to directly inform patient-important outcomes.36 Our review recognised this and hence did not report these surrogate outcomes. Finally, on the definition of sICH used in these RCTs,13–18 we chose the Heidelberg criteria for DIRECT-MT, DEVT and MR CLEAN-NO IV trials,13 15 16 and the Safe Implementation of Thrombolysis in Stroke–Monitoring Study (SITS–MOST) criteria for the SKIP trial,14 while for the SWIFT DIRECT and DIRECT SAFE17 18 trials we used their own trial-specific definitions (online supplemental appendices 2 and 6); the previous systematic review of four trials34 used Heidelberg criteria for DIRECT-MT and MR CLEAN-NO IV trials13 16 and National Institute of Neurological Disorders and Stroke (NINDS) criteria for SKIP and DEVT trials14 15 (online supplemental appendix 6); the previous systematic review of three trials35 used Heidelberg criteria for DIRECT-MT trials13 and NINDS criteria for SKIP and DEVT trials.14 15 Notwithstanding these differences in methods, our conclusion is essentially the same—there is little to no differences in outcomes with EVT alone compared with EVT plus alteplase.
On 3 February 2022, the European Stroke Organisation (ESO)–European Society for Minimally Invasive Neurological Therapy (ESMINT) published a guideline that made a strong recommendation in favour of intravenous thrombolysis plus mechanical thrombectomy over mechanical thrombectomy alone for patients who had an acute stroke presenting with anterior circulation large vessel occlusion and who are eligible for both treatments.37 Their associated evidence synthesis concluded moderate certainty evidence (due to inconsistency) for no difference in functional recovery without impairment or sICH, and high certainty evidence for no difference in mortality but greater chance of successful reperfusion with EVT plus alteplase. They rated down for inconsistency for recovery and sICH even though all CI in these forest plots overlapped and the I2 was 0% for both pooled effect estimates.
The difference in our appraisal of certainty of evidence is due to our approach of assessing imprecision. Specifically, we assessed values and preferences of patients presenting with acute stroke and found that most would consider a 1% absolute difference in functional recovery without impairment to be important. Accordingly, we judged the pooled effect for EVT alone versus combination therapy as imprecise as the 95% CI ranged from 5.4% more to 2.5% less recovering with no impairment; a range that includes both important benefits and harms associated with EVT alone and thus warranted rating down twice for imprecision according to the GRADE approach.38 The ESO–ESMINT guideline, alternatively, applied a non-inferiority margin of 1.3% and concluded that non-inferiority was not met and did not rate down for imprecision. The same issue affected the assessment of mortality. We viewed the associated 95% CI, which included a 2% decrease and a 4.9% increase in mortality with EVT alone, as including both important benefits and harms and so rated down two times for imprecision. The ESO–ESMINT guideline, again, did not consider this imprecise. The ESO–ESMINT guideline’s strong recommendation in favour of EVT plus alteplase appears to rest on significant effects on surrogate outcomes that favoured combination therapy; specifically, successful reperfusion and any intracranial haemorrhage. We did not include these outcomes in our review, and instead focused only on outcomes of direct important to patients: functional recovery, mortality and sICH.
Limitations
There are some limitations to our review. First, eligible trials used multiple criteria to define sICH. Based on feedback from our clinical experts, we chose the Heidelberg criteria for three trials,13 15 16 SITS–MOST criteria for the SKIP trial.14 The SWIFT DIRECT trial defined sICH as any parenchymal haematoma type 1, parenchymal haematoma type 2, remote intracranial haemorrhage, subarachnoid haemorrhage or intraventricular haemorrhage associated with a ≥4 point worsening on the National Institutes of Health Stroke Scale (NIHSS) at 24±6 hours post randomisation17 and the DIRECT SAFE trial defined sICH as NIHSS increase of 4 or more points at 24 hours window post stroke with ICH on CT scan18; the lack of statistical heterogeneity in our pooled estimate of effect (I2=0%) suggests our approach was valid. Second, although we found no difference in treatment effects between EVT with intravenous alteplase versus EVT alone, the associated estimates of precision included patient-important benefits and harms, which reduced our certainty of evidence to low or moderate. Third, our findings are only relevant to alteplase. Tenecteplase may be a more effective thrombolytic agent.39 40 If so, additional trials will be needed to determine whether the combination of tenecteplase and EVT is superior to EVT alone. Fourth, we relied on conference publications for two (SWIFT DIRECT and DIRECT SAFE)17 18 trials, and we contacted the lead investigators of each trial and confirmed the data presented at conferences.
Conclusions
Low certainty evidence suggests that there is possibly a small decrease in the proportion of patients that achieve functional independence and a small increase in mortality with EVT alone. Moderate certainty evidence suggests that there is probably a small decrease in sICH with EVT alone. The accompanying guideline provides contextualised guidance based on this body of evidence.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
References
Supplementary material
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors XW, ZY, JWB, GHG and ZA contributed to the conception of the work. XW, ZY, JWB, MDH, EES, KP, GHG, MPL and ZA contributed to the design of the work. RJC, XW, ZY, ZA, HY, YZ, YL, BT, XW and YW contributed to the acquisition, analysis and interpretation of data. XW, ZY, JWB, MDH, EES, GHG and ZA drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work. XW and ZY are joint primary authors.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.