Article Text
Abstract
STUDY OBJECTIVE To determine whether long term weight gain and weight loss are associated with subsequent risk of type 2 diabetes in overweight, non-diabetic adults.
DESIGN Prospective cohort. Baseline overweight was defined as BMI⩾27.3 for women and BMI⩾27.8 for men. Annual weight change (kg/year) over 10 years was calculated using measured weight at subjects' baseline and first follow up examinations. In the 10 years after measurement of weight change, incident cases of diabetes were ascertained by self report, hospital discharge records, and death certificates.
SETTING Community.
PARTICIPANTS 1929 overweight, non-diabetic adults.
MAIN RESULTS Incident diabetes was ascertained in 251 subjects. Age adjusted cumulative incidence increased from 9.6% for BMI<29 to 26.2% for BMI⩾37. Annual weight change over 10 years was higher in subjects who become diabetic compared with those who did not for all BMI<35. Relative to overweight people with stable weight, each kg of weight gained annually over 10 years was associated with a 49% increase in risk of developing diabetes in the subsequent 10 years. Each kg of weight lost annually over 10 years was associated with a 33% lower risk of diabetes in the subsequent 10 years.
CONCLUSIONS Weight gain was associated with substantially increased risk of diabetes among overweight adults, and even modest weight loss was associated with significantly reduced diabetes risk. Minor weight reductions may have major beneficial effects on subsequent diabetes risk in overweight adults at high risk of developing diabetes.
- obesity
- diabetes
Statistics from Altmetric.com
Evidence from several cohort studies indicates that weight gain during adulthood is associated with increased risk of type 2 diabetes.1-6 While this finding is present in most studies with only a few exceptions,7 8 it is not clear whether weight gain and weight loss are associated with changes in diabetes risk in overweight adults, a group at very high risk of developing diabetes. As the prevalence of overweight is rising among both adults and adolescents,9-11 it is important to understand whether people who are already overweight are susceptible to added diabetes risk after additional weight gain, and it is equally important to clarify the association between weight loss and subsequent diabetes risk in this high risk population. Accordingly, the purpose of this study is to test the hypotheses that weight gain over approximately 10 years in a cohort of overweight adults is associated with increased risk of diabetes in the subsequent 10 years, and that weight loss over 10 years is associated with lower risk of developing diabetes in the subsequent 10 years. We further hypothesised that diabetes risk associated with weight change is independent of other diabetes risk factors including age and baseline body mass index (BMI).
Methods
DATA AND STUDY SESIGN
Figure 1 is a schematic representation of the study design, timeline, data collection and measurements of interest in this investigation. The US First National Health and Nutrition Examination Survey, Epidemiologic Follow-up Study (NHEFS) is a prospective study of 14 407 subjects aged 25–74 at a baseline medical examination conducted from 1971–75. The baseline examination included measurement of height, weight, subscapular and triceps skinfold thicknesses, and systolic blood pressure. Baseline BMI was calculated from measured height and weight (kg/m2), and was used as an indicator of baseline obesity. The subscapular to triceps skinfold ratio was used as an indicator of centralised fat distribution.12 13Consistent with the United States' National Health Objectives outlined in Healthy People 2000, overweight was defined as BMI ⩾27.3 kg/m2 for women and BMI ⩾27.8 kg/m2 for men.14 The NHEFS has three follow up interviews, and weight was measured a second time at the 1982–84 interview. At both the baseline and first follow up interviews, subjects were asked how much exercise they received from recreational activities. Answers included “much”, “moderate” and “little or none.” Details of the design and operation of these surveys are described elsewhere.15-18
Study timeline, including collection of baseline measures, evaluation of weight change, and sources of incident diabetes cases, First National Health and Nutrition Examination Survey, Epidemiolgic Follow-up Study.
SELECTION CRITERIA
Of the 14 407 subjects in the NHEFS, 10 225 were excluded because they did not meet the criteria for baseline obesity. Of the remaining subjects, 639 died between baseline and first follow up, 994 were either diabetic at baseline or developed diabetes between baseline and first follow up, and an additional 688 people were excluded for one or more of the following reasons: loss to follow up, unknown diabetes status at the end of the study, pregnant when weight was measured, or missing weight measurements. The study sample consists of 1929 subjects. As expected, included subjects were younger, had lower systolic blood pressure and BMI, were more likely to be white, to have finished high school, and to have survived to the end of the study.
ASCERTAINMENT OF INCIDENT DIABETES CASES
Incident cases of diabetes were identified in three ways: from self report, subjects' health care facility records, and death certificates. At each follow up, subjects were asked if a doctor had told them they had diabetes since the last interview. Discharge diagnoses were collected from the health care facility records of subjects who reported staying overnight in a hospital or nursing home. These were coded according to the Ninth International Classification of Diseases, [codes 250.0–250.9].19 Death certificates were also collected and coded for decedents. A total of 251 cases of diabetes were identified, and 37% of these were confirmed by two or more sources of information.
STATISTICAL ANALYSIS
Because data from both the baseline and first follow up examinations were collected over a period of several years, the interval between subjects' weight measurements ranges from 7 to 12 years. Fifty four per cent of subjects had 10 or 11 years between weight measurements and 88% had between 8 and 11 years. The different intervals between weight measurements are adjusted for by the following formula, in which absolute weight change between baseline and first follow up is converted to average annual weight change during that period [Δ (kg)/Δ (years)]:

Annual weight change is a continuous variable that can be interpreted either as the mean yearly weight change (kg/y) between baseline and first follow up, or as the slope of a subject's weight trajectory throughout that period. Positive values reflect weight gain, and negative ones indicate weight loss. Total weight change can be approximated by multiplying annual weight change by 10.
In descriptive analyses, baseline BMI was categorised into several groups. Although somewhat arbitrary, these categories provide a basis for calculation of age standardised cumulative incidence of diabetes, and allow for presentation of descriptive associations between weight change and diabetes risk by strata of BMI, a confounding variable. Cumulative incidence of diabetes was age standardised by the direct method using the age distribution of the study sample as the reference population. We used the t test to examine differences in annual weight change between diabetic and non-diabetic subjects within body mass index group, and the χ2 test for trend (χ2 T) was used to examine linear associations between increasing tertile of annual weight change and age adjusted cumulative incidence of diabetes within BMI group. Tertiles of annual weight change (1: <−0.22 kg/y; 2: ⩽0.22 kg/y<0.39; 3: ⩾ 0.39 kg/y) were defined based on the sample distribution of this variable, which did not differ between men and women. The partial correlation coefficient (ρ) was used to determine the degree of age adjusted linear association between annual weight change and other continuous variables. The correlations are age adjusted because it is known that weight and weight change vary with age.20
The logistic regression model was used to examine the association between annual weight change and subsequent diabetes risk. Age, race, skinfold ratio, systolic blood pressure and education were considered potential confounders of the association between annual weight change and diabetes risk. Additionally, educational attainment (high school education compared with less than high school), was used as a proxy for socioeconomic status.21 As changes in physical activity may also be associated with weight change, we contrasted subjects who reported increases and decreases in physical activity during the period that weight change was measured, with subjects who reported no change in physical activity. Baseline BMI and annual weight change were both examined as continuous variables, and quadratic terms were tested to determine if the relationss between these variables and diabetes risk were non-linear.
The best multivariate models were chosen by the likelihood ratio test, and the Hosmer-Lemeshow test was used to evaluate the goodness of fit of the final model.22 To evaluate the predictive accuracy of the final model, we constructed a receiver operator curve contrasting the model's sensitivity to 1 minus its specificity. The area under the curve ranges from 0 to 1, and is represented numerically by the c statistic, with higher values indicating good predictive power of the model. All analyses were performed with SAS software, version 6.11.23 Using the adjusted parameter estimate for annual weight change from the final model, we predicted risk of diabetes associated with various levels of weight change.
Results
key points
-
Weight gain has been associated with risk of type 2 diabetes in adulthood.
-
It is not clear if weight gain further increases diabetes risk in overweight adults, a group already at high risk of diabetes.
-
Further weight gain was associated with significantly increased risk of diabetes in overweight adults, and even minor weight loss was associated with reduced risk.
-
Overweight adults should not be considered to have reached a threshold where additional weight gain fails to increase diabetes risk.
-
Weight loss interventions may be effective at reducing diabetes risk in overweight people.
Subjects' mean baseline BMI was 31.3 kg/m2, and these people gained an average of 0.1 kg/y (1.0 kg total) over the first 10 years of the study. Two thirds of the sample were women, and the crude incidence of diabetes was 13% (table 1).
Average annual weight change over 10 years and characteristics of 1929 overweight, non-diabetic1-150 subjects in the First National Health and Nutrition Examination Survey, Epidemiologic Follow up Study, 1971–1992
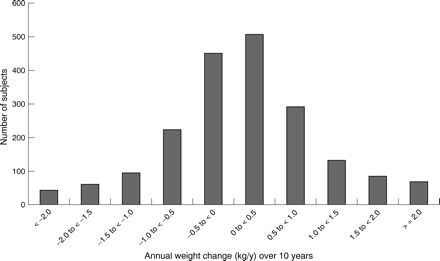
Annual weight change followed a normal distribution (fig 2), and the median value was 0.09 kg/y, indicating that a majority of subjects gained weight in the 10 years between baseline and first follow up. However, annual weight change was highly variable, did not differ by sex, and was inversely correlated with baseline BMI (partial ρ= −0.27; p<0.001). When the highest BMI category (BMI⩾37) was excluded, the inverse correlation between age adjusted annual weight change and BMI was substantially reduced, but was still statistically significant because of the large sample (partial ρ= −0.11; p<0.001). Subjects who reported no change in physical activity and those who reported decreased physical activity between baseline and first follow up both gained weight during that period (median weight change: 0.10 and 0.15 kg/y, respectively). Subjects who reported increased physical activity were weight stable (median weight change: 0.04 kg/y).
Distribution of annual weight change over 10 years in a cohort of 1929 overweight adults.
In the 10 years after measurement of weight change, the age standardised cumulative incidence of diabetes was 15.5%, and diabetes incidence increased with increasing baseline BMI (table 2). While nearly 10% of subjects in the lowest BMI group developed diabetes, over 26% of subjects in the highest group developed diabetes. On average, both diabetic and non-diabetic subjects in all but the highest BMI group either gained weight, or maintained relatively stable weight during follow up (fig 3). In contrast, both diabetic and non-diabetic subjects in the highest BMI group lost weight. For BMI<31 and 33⩽BMI<35, subjects who developed diabetes had significantly higher annual weight change in the previous 10 years compared with subjects who did not develop diabetes. For instance, for BMI<29, total weight gain (annual weight change × 10) was (mean (SD) 5.6 (8.5) kg for subjects who subsequently developed diabetes, and 2.2 (8.6) kg for those who did not (p=0.004). For 29⩽BMI<31, total weight gain was 5.2 (8.6) kg for subjects who developed diabetes and 0.9 (8.1) kg for those who did not (p<0.001). A significant difference in annual weight change was also observed in the 33⩽BMI<35 group. Annual weight change was higher in diabetic subjects in the remaining BMI groups, but these differences were not statistically significant.
Distribution of incident2-150 cases of diabetes, by baseline body mass index
Annual weight change over 10 years in relation to development of diabetes in the subsequent 10 years, by baseline BMI. Comparison is shown between subjects who developed diabetes and those who did not. p Value is for the t test of differences in means between these groups.
Increasing tertile of annual weight change was associated with increased age adjusted risk of diabetes at most levels of baseline BMI (fig 4). Significant trends were observed between tertile of annual weight change and diabetes risk for BMI<29 (p=0.003, χ2 T ), 29⩽BMI<31 (p=0.005, χ2 T ), and 35⩽BMI<37 (p=0.04, χ2 T). This trend reached conventional statistical significance for 33⩽BMI<35 (p=0.05, χ2 T). The greatest cumulative incidence of diabetes was observed in the third tertile of annual weight change in all but the highest BMI group.
Ten year cumulative incidence of diabetes in relation to annual weight change in the previous 10 years and baseline BMI among 1929 overweight adults. p Value is for the age adjusted χ2 test for trend between increasing tertile of annual weight change over 10 years and diabetes risk in the subsequent 10 years.
We examined whether the association between annual weight change and subsequent diabetes risk would persist after adjustment for multiple diabetes risk factors, and we were particularly interested in whether the addition of risk variables other than baseline age and BMI would reduce or eliminate the association between annual weight change and diabetes risk. Accordingly, two models were defined (table 3). Model I included age, age2 and BMI. The quadratic term for age indicates a non-linear association between age and diabetes risk. Model II included all variables in model I as well as sex, race, education, systolic blood pressure, skinfold ratio and reported change in physical activity. Regression analyses indicated that annual weight change was a significant predictor of subsequent diabetes risk in model I: odds ratio=1.46, 95% confidence intervals =1.26, 1.69. The strength of the association between annual weight change and diabetes risk was virtually unchanged after adjustment for the additional risk variables in model II: odds ratio=1.49, 95% confidence intervals = 1.29, 1.73.
Logistic regression analysis of the effect of average annual weight change over 10 years and baseline characteristics on risk of subsequent diabetes in a cohort of overweight adults, First National Health and Nutrition Examination Survey, Epidemiologic Follow up Study, 1971–1992
Model II indicated that race, sex, education and change in physical activity did not predict of subsequent diabetes risk in this overweight cohort. There was no evidence of interaction between annual weight change and the covariates, and there was no quadratic relation between either BMI or annual weight change with diabetes risk. The Hosmer-Lemeshow test indicated that model II fits the data well (p=0.82). The area under the ROC curve for model II was 0.68, indicating relatively good predictive power.
We estimated from model II odds ratios for specific levels of annual weight change, and corresponding total weight change over 10 years (table 4). Relative to no weight change, weight gain was associated with increased risk of developing diabetes. Model estimates suggested significant increases in diabetes risk after minor weight gain. Risk estimates ranged from 1.04 (95% confidence intervals =1.03, 1.06) for a 10 year weight gain of 1 kg to 2.22 (95% confidence intervals =1.66, 2.98) for a 10 year weight gain of 20 kg. Conversely, our model indicated that weight loss was associated with significant reductions in subsequent diabetes risk relative to no weight change. A weight loss of 1 kg over 10 years was associated with a slight, but significantly decreased subsequent risk of developing diabetes: odds ratio=0.96, 95% confidence intervals =0.95, 0.97. Greater weight loss was associated with even larger reductions in subsequent diabetes risk. A weight loss of 10 kg over 10 years was associated with a 33% reduction in subsequent diabetes risk compared with no weight loss: odds ratio=0.67, 95% confidence intervals = 0.58, 0.78. These associations were independent of age, baseline BMI, and other risk variables.
Average annual weight change over 10 years, absolute weight change over 10 years, and estimated risk of diabetes risk in a cohort of overweight adults, First National Health and Nutrition Examination Survey, Epidemiologic Follow-up Study, 1971–1992
Discussion
Our findings extend previous work on the association between weight change and diabetes risk by demonstrating a significant association between weight gain and increased risk of diabetes in a sample of 1929 adults who are already overweight, a group at especially high risk of developing diabetes. Our findings suggest that people who are overweight are susceptible to added diabetes risk after additional weight gain. This finding is in agreement with previous studies in special populations and one in a national sample showing that weight gain in adulthood is associated with increased risk of developing diabetes.1-6 However, our findings related to weight gain and subsequent diabetes risk from this large sample apply specifically to men and women whose overweight already places them in a high risk category. The importance of this finding is that it suggests that many overweight people have not reached a threshold at which additional weight gain fails to increase diabetes risk.
Our findings also offer important prospective evidence that overweight people who lose even minor amounts of weight may have less risk of developing diabetes relative to overweight people who are weight stable. Of particular note, our estimates indicated that a loss of 10 kg over 10 years was associated with a 33% reduction in diabetes in the subsequent 10 years, relative to no weight change. This finding suggests that achievement of relatively modest weight loss goals in overweight people may result in substantial reductions in diabetes risk. This finding is consistent with results of smaller studies: a previous investigation of 21 overweight, non-diabetic men (mean body mass index 29.7), showed that relatively minor weight loss (mean 8.1 kg) accompanied by a modest exercise programme resulted in significant reductions in fasting insulin, percent body fat and waist-hip ratio, indicating general improvement in diabetes risk profiles for these high risk men.24 Similarly, in a study of 48 overweight, non-diabetic, postmenopausal women (mean BMI 32.7), modest weight loss (mean 9.8 kg) resulted in significant improvements in lipid profiles, indicating a reduction in cardiovascular risk.25 Our finding of a long term reduction in diabetes risk associated with modest weight loss in this large cohort of overweight adults has broad clinical and public health implications because the prevalence of overweight in the United States continues to increase,9-11 and because, as we showed, overweight people often continue to gain weight.
The United States' Diabetes Prevention Program, a clinical trial designed to determine if diabetes can be prevented or delayed in persons with impaired glucose tolerance (a condition associated with overweight), includes a group randomised to diet and exercise, two factors associated with weight loss. Our results concerning weight loss and reduced diabetes risk may be an early indication of the effects of non-pharmacological interventions on diabetes risk in a large sample of high risk people. In our study, subjects who reported no change in physical activity and those who reported decreased physical activity between baseline and first follow up both gained weight, while those who reported increased physical activity remained weight stable. Despite the association between physical activity and improved diabetes risk profiles in previous studies,24 25 our models did not indicate a significant association between increases in reported physical activity and subsequent diabetes risk. However, the measure of physical activity in this observational study is crude and applied only to recreational activities. It is not surprising that previous studies with exercise interventions showed positive effects of physical activity on diabetes risk factors. Availability of better data on changes in physical activity may have provided greater insight into how this factor is associated with long term weight change and diabetes risk in overweight people.
Although the age standardised cumulative incidence of diabetes in the BMI⩾37 kg/m2 group (n=141) was 26.2%, annual weight change was unrelated to development of diabetes in this group. This finding suggests that it is perhaps only at the highest levels of obesity that the effect of weight gain reaches a threshold beyond which this factor does not contribute added diabetes risk. It was therefore not surprising that when we repeated our regression analyses excluding the highest BMI group, the magnitude of association between annual weight change and diabetes risk increased. The observation that both diabetic and non-diabetic subjects in the highest baseline BMI category tended to lose weight may be “regression to the mean”, rather then a biological phenomenon.26 Although there was an inverse correlation between baseline BMI and annual weight change, this association was weak across lower BMI groups. Moreover, significant differences in annual weight change between diabetic and non-diabetic subjects were observed in most of the lower BMI groups, supporting the idea that annual weight change is most useful in predicting subsequent diabetes risk in the less extreme range of overweight, which constituted 93% of this cohort.
While the ROC curve indicated relatively good predictive power of the final model, it also suggested that factors other than those in our model are needed to accurately predict diabetes in overweight people. Addition of key baseline measures, such as fasting glucose and visceral fat may have resulted in greater predictive power of the model. The absence of repeated fasting glucose measures throughout the study probably contributed to under-identification of incident cases, which also may have limited the model's predictive accuracy. However, the validity of our results rests on an accurate description of weight change before development of diabetes. Therefore, under-identification of incident cases would not have changed our findings as there is no reason to believe that the weight histories of undiagnosed and diagnosed diabetic people differ. This study is also limited by the availability of only two measured weights taken 10 years apart, precluding examination of potentially important effects of weight cycling that may have occurred in the interim period. Other potentially important factors such as family history of both diabetes and overweight were not available in the NHEFS, but these may be critical to consider in future studies of diabetes in high risk populations.
It is important to emphasise that while our data allowed for identification of diabetes cases from three sources of information, no data on fasting or post-challenge glucose were available. The latter measures are standard screening instruments for detection of undiagnosed diabetes. Absence of these measures in the NHEFS presumably resulted in misclassification of diabetic people as non-diabetic, although it is difficult to speculated on the degree and effect of this misclassification.
Duration of overweight predicts diabetes risk,27suggesting that this factor may have cumulative effects. To determine if the association between annual weight change and diabetes risk may have been attributable to overweight beginning earlier in life for subjects who became diabetic relative to those who did not, we examined diabetes status at the end of the study in relation to self reported weight at age 25. There was no difference in mean reported weight at age 25 between subjects who became diabetic and those who did not, after adjusting for sex, race, and baseline age. Although weight at age 25 is an imperfect tool for evaluating duration of overweight before entry into the study, it does provide evidence that overweight subjects in this study had comparable weights in early adulthood, and supports the role of weight gain as a risk factor for diabetes in this overweight cohort.
This study raises interesting questions about mechanisms that make some overweight people more likely to develop diabetes than others. It is known that overweight is associated with insulin resistance through a variety of mechanisms, but many overweight people with insulin resistance never develop frank diabetes, while, over time, others develop a relative insulin deficiency leading to diabetes. This observation is suggestive of biological heterogeneity among overweight people. Further investigation of factors that distinguish high risk from low risk overweight people will become increasingly important as the prevalence of overweight continues to rise, placing more people at risk of developing diabetes. At the present time, weight loss seems to be an effective way to reduce diabetes risk in overweight adults.
In summary, our results indicate that weight gain was associated with increased diabetes risk in overweight adults and that even modest weight loss in overweight people was associated with reduced risk of developing diabetes. Weight loss interventions may be effective in reducing long term diabetes risk even among overweight people.
Acknowledgments
The authors thank Drs Patricia Peyser and MaryFran Sowers for helpful comments on an earlier draft of this manuscript. The data used in this study were collected by the National Center for Health Statistics, Bethesda MD. Parts of this work were presented at the annual meeting of the American Diabetes Association, Chicago, IL, June, 1998.
References
Footnotes
-
Funding: this research was supported by Multidisciplinary Training Grant in Aging (no AG-00114) from the National Institute on Aging, an AARP/Andrus Foundation Graduate Fellowship, and a Blue Cross and Blue Shield of Michigan Student Award Program Grant (no 275-SAP/97) to Dr Resnick.
-
Conflicts of interest: none.