-
PDF
- Split View
-
Views
-
Cite
Cite
S. Tal, V. Guller, Y. Shavit, F. Stern, S. Malnick, Mortality predictors in hospitalized elderly patients, QJM: An International Journal of Medicine, Volume 104, Issue 11, November 2011, Pages 933–938, https://doi.org/10.1093/qjmed/hcr093
Close - Share Icon Share
Abstract
Aim: To find out which of the two predictors, Charlson co-morbidity index or vitamin B12, better estimates the risk of in-hospital mortality in seriously ill patients.
Method: Electronic hospital records of 1509 elderly patients aged 65 and older were retrospectively surveyed.
Results: Albumin, age and elevated vitamin B12 levels were significantly associated with increased in-hospital mortality. Charlson co-morbidity index was not significantly associated with death. The highest mortality (24.3%) was found in the group of patients who were concomitantly in the lowest albumin quartile and the highest vitamin B12 levels quartile. In this group, mortality increased significantly with age. By elasticity calculation, vitamin B12 capability to predict mortality was higher by ∼3 times than that of Charlson co-morbidity index.
Conclusions: In view of the fact that vitamin B12 levels have been found to predict mortality, they should be measured in geriatric practice, in addition to albumin levels, as a practical and reliable tool for identifying high risk elderly hospitalized patients. Probably, a combination of two or more available and inexpensive routinely taken tests can give a better estimation of mortality than some complicated tools, like Charlson co-morbidity index.
Introduction
To classify prognostic comorbidity in critically ill patients, some methods are used, but none of them has been set as a gold standard for this purpose. The main significance of these tests is in helping physicians in giving priority to certain medical resources. A widely used and accepted test is the Charlson co-morbidity index.1 It is a weighted index based on a mathematical model, which takes into account the number and the severity of comorbid diseases. This test is a readily applicable and valid method of estimating risk of death from co-morbid disease in medical patients.2,3 At least nine studies, comprising more than 30 000 patients, have validated the Charlson index in a wide variety of diseases for numerous clinical outcomes.3 The Charlson co-morbidity index is commonly used for risk adjustment in outcome assessment in critical care health services research.4 It has been found, in a study of 62 456 inpatients, including elderly patients, that it is a useful approach for risk adjustment.5
Recently, a linear correlation between mortality and vitamin B12 levels was unpredictably found in 1570 hospitalized elderly aged 65 and over admitted to internal and geriatrics departments. Patients with malignancy and chronic liver disease were excluded, as it is well known that a high vitamin B12 level is a marker for mortality in such patients.6 Some studies have found a link between high vitamin B12 levels and higher risk of mortality in frail elderly patients with no malignancy.7–12 There are, however, two studies13,14 that have not found a correlation between high vitamin B12 levels and a higher risk of mortality. Contrary to the above studies,6–11 these two studies13,14 were conducted in unhospitalized elderly for 7.6 and 6 years, respectively, and vitamin B12 levels were determined at the beginning of the study and not near the time of death.
In the present study we have tried to find out which of the two predictors, Charlson co-morbidity index or vitamin B12 level, better estimates the risk of mortality in hospitalized seriously ill patients aged 65 and over.
Method
Study population and design
Electronic hospital records (n = 13 000) of elderly patients aged 65 and older admitted during 2007 to the four internal medicine departments and the geriatrics department at Kaplan Medical Center (KMC) Rehovot, Israel, were retrospectively surveyed. Only patients who had been tested for vitamin B12 levels on admission were included in the study. Exclusion criteria were treatment with orally or parenterally administered vitamin B12, chronic liver disease, malignancy, small bowel disease (celiac and Crohn's disease), and previous gastric and colon surgery. One thousand eight hundred patients who had not been treated with vitamin B12 and had been tested for vitamin B12 levels on admission were considered for the study. Two hundred ninety one of the 1800 patients were excluded from the study: 180 with cancer, 48 with liver disease, and 2 with inflammatory bowel diseases and 61 patients with incomplete records—leaving 1509 patients for the study. The recorded data included patient demographics, medical diagnoses, laboratory results and clinical outcomes. For each patient Charlson co-morbidity index score was calculated. Vitamin B12 was assayed at the KMC laboratory, using the ADVIA Centaur vitamin B12 assay (ADVIA Centaur, Siemens, Tarrytown, NY), which is a competitive immunoassay using direct chemiluminescent technology. The KMC ethics committee approved the study.
Charlson co-morbidity index
The Charlson co-morbidity index assigns non-zero weights to 19 comorbid conditions (usually limited to chronic conditions). The severity weights can take on values of 1, 2, 3 or 6 depending on the disease severity and the mortality risk. Patient score is estimated by a weighted calculation of comorbid diseases and their severity. The index is calculated by summing up the individual scores.15
Statistical analysis
The SPSS 13.0 program was used for statistical analysis (SPSS, Inc., Chicago, IL). Univariant statistical methods were used in order to analyze differences between study groups (dead vs. alive) and population characteristics. T-tests were performed for consecutive variables and chi-square tests for dichotomous variables. The independent variables were entered to the logistic regression models according to their statistical significance level in the univariant analysis.
For analyzing the impact of independent variables on mortality two logistic regression models were used. In the first model the variable vitamin B12 had been transformed to a natural logarithm (ln) and in the second model it was entered into the regression linearly. The estimated coefficients of the regression of the second model were used to calculate the elasticity between independent variables and the probability of mortality. Elasticity is an indicator of sensitivity of the probability of mortality to change with the independent variable. Elasticity is the ratio between percent change in mortality (y) and percent change in each independent variable (x).
To evaluate the mutual impact of albumin and vitamin B12 levels on mortality, they were divided into quartiles. The albumin quartiles (Q) are g/l: Q1 = 1.5−3.2; Q2 = 3.3−3.5; Q3 = 3.6−3.9; Q4 = 4.0−5.0. The vitamin B12 quartiles are pmol/l: Q1 = 57−231; Q2 = 232−352; Q3 = 353−512; Q4 = 513−1445.
Results
The characteristics of the 1509 patients (62.2% female) are presented in Table 1. Their mean age was 81.5. Women were older than men (81.8 vs. 80.9, P = 0.007) and consumed significantly more psychiatric medications (0.7 ± 0.9 vs. 0.6, P = 0.017) (data not shown). Mean serum vitamin B12 concentration of the study population was 414.4 pmol/l. Mean Charlson co-morbidity index score was 1.3 (Table 1).
Baseline patient characteristics (n = 1509)
| Characteristic . | . |
|---|---|
. | |
| Age (mean ± SD) | 81.5 ± 7.1 |
| Diabetes mellitus (%) | 35 |
| Men (%) | 38 |
| Mortality (%) | 6.2 |
| Charlson index (mean ± SD) | 1.3 ± 1.3 |
| Number of medications (mean ± SD) | 5.7 ± 2.8 |
| Laboratory values (mean ± SD) | |
| Hemoglobin (g/dl) | 11.7 ± 1.8 |
| Mean corpuscular volume (fl) | 86.7 ± 6.5 |
| Albumin (mg/dl) | 3.6 ± 0.5 |
| Vitamin B12 (pmol/l) | 414 ± 260 |
| Characteristic . | . |
|---|---|
. | |
| Age (mean ± SD) | 81.5 ± 7.1 |
| Diabetes mellitus (%) | 35 |
| Men (%) | 38 |
| Mortality (%) | 6.2 |
| Charlson index (mean ± SD) | 1.3 ± 1.3 |
| Number of medications (mean ± SD) | 5.7 ± 2.8 |
| Laboratory values (mean ± SD) | |
| Hemoglobin (g/dl) | 11.7 ± 1.8 |
| Mean corpuscular volume (fl) | 86.7 ± 6.5 |
| Albumin (mg/dl) | 3.6 ± 0.5 |
| Vitamin B12 (pmol/l) | 414 ± 260 |
Baseline patient characteristics (n = 1509)
| Characteristic . | . |
|---|---|
. | |
| Age (mean ± SD) | 81.5 ± 7.1 |
| Diabetes mellitus (%) | 35 |
| Men (%) | 38 |
| Mortality (%) | 6.2 |
| Charlson index (mean ± SD) | 1.3 ± 1.3 |
| Number of medications (mean ± SD) | 5.7 ± 2.8 |
| Laboratory values (mean ± SD) | |
| Hemoglobin (g/dl) | 11.7 ± 1.8 |
| Mean corpuscular volume (fl) | 86.7 ± 6.5 |
| Albumin (mg/dl) | 3.6 ± 0.5 |
| Vitamin B12 (pmol/l) | 414 ± 260 |
| Characteristic . | . |
|---|---|
. | |
| Age (mean ± SD) | 81.5 ± 7.1 |
| Diabetes mellitus (%) | 35 |
| Men (%) | 38 |
| Mortality (%) | 6.2 |
| Charlson index (mean ± SD) | 1.3 ± 1.3 |
| Number of medications (mean ± SD) | 5.7 ± 2.8 |
| Laboratory values (mean ± SD) | |
| Hemoglobin (g/dl) | 11.7 ± 1.8 |
| Mean corpuscular volume (fl) | 86.7 ± 6.5 |
| Albumin (mg/dl) | 3.6 ± 0.5 |
| Vitamin B12 (pmol/l) | 414 ± 260 |
Ninety three (6.2%) patients died during hospitalization (Table 2). Univariant statistical analysis showed significant differences between patients still alive and those who had died (Table 2). The patients who had died were significantly older than the survivors (84.8 vs. 81.3). Vitamin B12 levels and the Charlson co-morbidity index score of the patients who had died were significantly higher (597.8 vs. 402.3 pmol/l and 1.6 vs. 1.2, respectively). Their serum albumin levels were significantly lower (2.85 vs. 3.59 mg/dl), and they consumed a lower number of medications (5.0 vs. 5.7).
Study population characteristics by mortality
| Characteristic . | Dead (n = 93) . | Alive (n = 1416) . | P-value . |
|---|---|---|---|
. | |||
| Age (mean ± SD) | 84.8 ± 8.0 | 81.3 ± 7.0 | <0.001 |
| Diabetes mellitus (%) | 31 | 36 | |
| Men (%) | 42 | 37.5 | |
| Charlson index (mean ± SD) | 1.6 ± 1.4 | 1.2 ± 1.3 | 0.003 |
| Number of medications (mean ± SD) | 5.0 ± 2.7 | 5.7 ± 2.9 | 0.01 |
| Metformin (%) | 9.7 | 17.1 | 0.063 |
| Angiotensin converting enzyme Inhibitors (%) | 28 | 42 | 0.01 |
| Laboratory values (mean ± SD) | |||
| Albumin (mg/dl) | 2.9 ± 0.6 | 3.6 ± 0.5 | <0.001 |
| Vitamin B12 (pmol/l) | 598 ± 302 | 402 ± 253 | <0.001 |
| Characteristic . | Dead (n = 93) . | Alive (n = 1416) . | P-value . |
|---|---|---|---|
. | |||
| Age (mean ± SD) | 84.8 ± 8.0 | 81.3 ± 7.0 | <0.001 |
| Diabetes mellitus (%) | 31 | 36 | |
| Men (%) | 42 | 37.5 | |
| Charlson index (mean ± SD) | 1.6 ± 1.4 | 1.2 ± 1.3 | 0.003 |
| Number of medications (mean ± SD) | 5.0 ± 2.7 | 5.7 ± 2.9 | 0.01 |
| Metformin (%) | 9.7 | 17.1 | 0.063 |
| Angiotensin converting enzyme Inhibitors (%) | 28 | 42 | 0.01 |
| Laboratory values (mean ± SD) | |||
| Albumin (mg/dl) | 2.9 ± 0.6 | 3.6 ± 0.5 | <0.001 |
| Vitamin B12 (pmol/l) | 598 ± 302 | 402 ± 253 | <0.001 |
Study population characteristics by mortality
| Characteristic . | Dead (n = 93) . | Alive (n = 1416) . | P-value . |
|---|---|---|---|
. | |||
| Age (mean ± SD) | 84.8 ± 8.0 | 81.3 ± 7.0 | <0.001 |
| Diabetes mellitus (%) | 31 | 36 | |
| Men (%) | 42 | 37.5 | |
| Charlson index (mean ± SD) | 1.6 ± 1.4 | 1.2 ± 1.3 | 0.003 |
| Number of medications (mean ± SD) | 5.0 ± 2.7 | 5.7 ± 2.9 | 0.01 |
| Metformin (%) | 9.7 | 17.1 | 0.063 |
| Angiotensin converting enzyme Inhibitors (%) | 28 | 42 | 0.01 |
| Laboratory values (mean ± SD) | |||
| Albumin (mg/dl) | 2.9 ± 0.6 | 3.6 ± 0.5 | <0.001 |
| Vitamin B12 (pmol/l) | 598 ± 302 | 402 ± 253 | <0.001 |
| Characteristic . | Dead (n = 93) . | Alive (n = 1416) . | P-value . |
|---|---|---|---|
. | |||
| Age (mean ± SD) | 84.8 ± 8.0 | 81.3 ± 7.0 | <0.001 |
| Diabetes mellitus (%) | 31 | 36 | |
| Men (%) | 42 | 37.5 | |
| Charlson index (mean ± SD) | 1.6 ± 1.4 | 1.2 ± 1.3 | 0.003 |
| Number of medications (mean ± SD) | 5.0 ± 2.7 | 5.7 ± 2.9 | 0.01 |
| Metformin (%) | 9.7 | 17.1 | 0.063 |
| Angiotensin converting enzyme Inhibitors (%) | 28 | 42 | 0.01 |
| Laboratory values (mean ± SD) | |||
| Albumin (mg/dl) | 2.9 ± 0.6 | 3.6 ± 0.5 | <0.001 |
| Vitamin B12 (pmol/l) | 598 ± 302 | 402 ± 253 | <0.001 |
Table 3 presents the results of the regression analysis of the first model. An increase of one unit in ln(vitamin B12) increases mortality by 2.3. An increase of one unit in ln(vitamin B12) at vitamin B12 mean level (414 pmol/l) leads to an increase of 682 units of vitamin B12 (ln414 = 6 and ln1096 = 7).
The impact of independent variables on mortality by the first model of logistic regression
| Variable . | beta . | SE . | P-value . | OR (95% CI) . |
|---|---|---|---|---|
. | ||||
| Ln(vitamin B12) | 0.842 | 0.208 | <0.001 | 2.320 (1.545–3.484) |
| Albumin | −2.335 | 0.244 | <0.001 | 0.097 (0.060–0.156) |
| Age | 0.049 | 0.017 | 0.004 | 1.050 (1.016–1.085) |
| Charlson index | 0.162 | 0.093 | 0.083 | 1.176 (0.979–1.412) |
| Number of medications | −0.075 | 0.047 | 0.106 | 0.927 (0.846–1.016) |
| Metformin | 0.027 | 0.409 | 0.947 | 1.028 (0.461–2.290) |
| Constant | −4.110 | 2.046 | 0.045 | 0.016 |
| Variable . | beta . | SE . | P-value . | OR (95% CI) . |
|---|---|---|---|---|
. | ||||
| Ln(vitamin B12) | 0.842 | 0.208 | <0.001 | 2.320 (1.545–3.484) |
| Albumin | −2.335 | 0.244 | <0.001 | 0.097 (0.060–0.156) |
| Age | 0.049 | 0.017 | 0.004 | 1.050 (1.016–1.085) |
| Charlson index | 0.162 | 0.093 | 0.083 | 1.176 (0.979–1.412) |
| Number of medications | −0.075 | 0.047 | 0.106 | 0.927 (0.846–1.016) |
| Metformin | 0.027 | 0.409 | 0.947 | 1.028 (0.461–2.290) |
| Constant | −4.110 | 2.046 | 0.045 | 0.016 |
R2 = 0.32. OR: Odds ratio.
The impact of independent variables on mortality by the first model of logistic regression
| Variable . | beta . | SE . | P-value . | OR (95% CI) . |
|---|---|---|---|---|
. | ||||
| Ln(vitamin B12) | 0.842 | 0.208 | <0.001 | 2.320 (1.545–3.484) |
| Albumin | −2.335 | 0.244 | <0.001 | 0.097 (0.060–0.156) |
| Age | 0.049 | 0.017 | 0.004 | 1.050 (1.016–1.085) |
| Charlson index | 0.162 | 0.093 | 0.083 | 1.176 (0.979–1.412) |
| Number of medications | −0.075 | 0.047 | 0.106 | 0.927 (0.846–1.016) |
| Metformin | 0.027 | 0.409 | 0.947 | 1.028 (0.461–2.290) |
| Constant | −4.110 | 2.046 | 0.045 | 0.016 |
| Variable . | beta . | SE . | P-value . | OR (95% CI) . |
|---|---|---|---|---|
. | ||||
| Ln(vitamin B12) | 0.842 | 0.208 | <0.001 | 2.320 (1.545–3.484) |
| Albumin | −2.335 | 0.244 | <0.001 | 0.097 (0.060–0.156) |
| Age | 0.049 | 0.017 | 0.004 | 1.050 (1.016–1.085) |
| Charlson index | 0.162 | 0.093 | 0.083 | 1.176 (0.979–1.412) |
| Number of medications | −0.075 | 0.047 | 0.106 | 0.927 (0.846–1.016) |
| Metformin | 0.027 | 0.409 | 0.947 | 1.028 (0.461–2.290) |
| Constant | −4.110 | 2.046 | 0.045 | 0.016 |
R2 = 0.32. OR: Odds ratio.
The risk of mortality increased with lower albumin levels and with advanced age (Table 3). There is a linear inverse and significant correlation between lower albumin levels (Q1 and Q2) and mortality (P < 0.0001), data not shown. Mortality was not associated significantly with Charlson co-morbidity index score, the number of medications and metformin consumption (Table 3).
In the second model, the highest elasticity was found for albumin (Table 4). Thus, at an average value of the variables, an increase in one percent in albumin brings about a decrease of 8.1% in mortality. An increase in one percent in vitamin B12 prompts an increase of 0.53% in mortality, whereas an increase in one percent in the Charlson co-morbidity index prompts an increase of 0.19% in mortality. Thus, vitamin B12 capability to predict mortality is higher by ∼3 times than that of Charlson co-morbidity index.
The impact of independent variables on mortality expressed by elasticity by the second model of logistic regression
| Variable . | Elasticity (SE) . | P-value . |
|---|---|---|
. | ||
| Albumin | −8.156 (0.868) | 0.000 |
| Age | 3.964 (1.353) | 0.003 |
| Vitamin B12 | 0.5397 (0.1498) | 0.000 |
| Charlson index | 0.196 (0.1141) | 0.085 |
| Number of medications | −0.3929 (0.256) | 0.1256 |
| Metformin | −0.00126 (0.0659) | 0.985 |
| Variable . | Elasticity (SE) . | P-value . |
|---|---|---|
. | ||
| Albumin | −8.156 (0.868) | 0.000 |
| Age | 3.964 (1.353) | 0.003 |
| Vitamin B12 | 0.5397 (0.1498) | 0.000 |
| Charlson index | 0.196 (0.1141) | 0.085 |
| Number of medications | −0.3929 (0.256) | 0.1256 |
| Metformin | −0.00126 (0.0659) | 0.985 |
SE = Standard error.
The impact of independent variables on mortality expressed by elasticity by the second model of logistic regression
| Variable . | Elasticity (SE) . | P-value . |
|---|---|---|
. | ||
| Albumin | −8.156 (0.868) | 0.000 |
| Age | 3.964 (1.353) | 0.003 |
| Vitamin B12 | 0.5397 (0.1498) | 0.000 |
| Charlson index | 0.196 (0.1141) | 0.085 |
| Number of medications | −0.3929 (0.256) | 0.1256 |
| Metformin | −0.00126 (0.0659) | 0.985 |
| Variable . | Elasticity (SE) . | P-value . |
|---|---|---|
. | ||
| Albumin | −8.156 (0.868) | 0.000 |
| Age | 3.964 (1.353) | 0.003 |
| Vitamin B12 | 0.5397 (0.1498) | 0.000 |
| Charlson index | 0.196 (0.1141) | 0.085 |
| Number of medications | −0.3929 (0.256) | 0.1256 |
| Metformin | −0.00126 (0.0659) | 0.985 |
SE = Standard error.
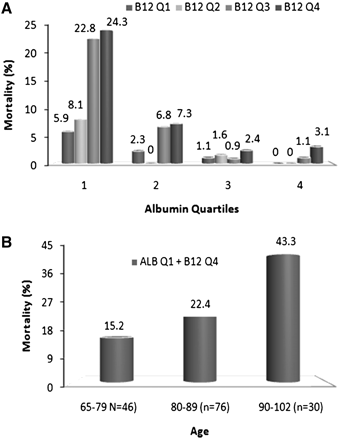
The highest mortality (24.3%) was found in the group of patients who were concomitantly in the lowest albumin quartile (Q1) and the highest vitamin B12 quartile (Q4). In this group mortality increased with age from 15.2% in the patients aged 65–79 up to 43.3%, in patients aged 90–102 (Figure 1A and B). The difference between the three age groups in this group of patients is significant (P < 0.007), Figure 1B. There is a significant difference between the highest vitamin B12 quartile and the three other quartiles of vitamin B12 within Q1 of albumin (P < 0.0001). In Q2 of albumin quite similar interaction was found (P = 0.03). In Q3 and Q4 of albumin this interaction between albumin and vitamin B12 is insignificant (Figure 1A). Number of patients for the different combinations of the albumin quartiles and the vitamin B12 quartiles are given in Table 5
(A) Mortality rate by albumin and vitamin B12 quartiles. (B) Mortality rate in the combined lowest albumin and the highest vitamin B12 quartiles by age groups.
Number of patients for the different combinations of the albumin quartiles and the vitamin B12 quartiles
| Vitamin B12 quartiles . | Albumin quartiles . | |||
|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | |
. | ||||
| 1 | 85 | 86 | 123 | 86 |
| 2 | 74 | 64 | 124 | 112 |
| 3 | 101 | 74 | 109 | 91 |
| 4 | 152 | 82 | 82 | 64 |
| Vitamin B12 quartiles . | Albumin quartiles . | |||
|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | |
. | ||||
| 1 | 85 | 86 | 123 | 86 |
| 2 | 74 | 64 | 124 | 112 |
| 3 | 101 | 74 | 109 | 91 |
| 4 | 152 | 82 | 82 | 64 |
Number of patients for the different combinations of the albumin quartiles and the vitamin B12 quartiles
| Vitamin B12 quartiles . | Albumin quartiles . | |||
|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | |
. | ||||
| 1 | 85 | 86 | 123 | 86 |
| 2 | 74 | 64 | 124 | 112 |
| 3 | 101 | 74 | 109 | 91 |
| 4 | 152 | 82 | 82 | 64 |
| Vitamin B12 quartiles . | Albumin quartiles . | |||
|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | |
. | ||||
| 1 | 85 | 86 | 123 | 86 |
| 2 | 74 | 64 | 124 | 112 |
| 3 | 101 | 74 | 109 | 91 |
| 4 | 152 | 82 | 82 | 64 |
Discussion
The study is based on patient on-admission electronic data. Demographics, admission diagnosis, comorbid diseases, medications and laboratory information are often the factors most readily available from administrative databases. Each of these factors might influence prognosis to a varying degree.16
Low level of albumin was found to have a significant impact on mortality, consistent with many other studies.10,17–23 Low level of albumin is often associated with malnutrition. The association between higher mortality rates and malnutrition is a common condition among hospitalized older patients. Poor nutritional condition has already been established as an independent predictor of in-hospital mortality.21 It is not clear to what extent albumin is a marker of nutritional status or of more complex disease processes. Little is known about whether the lower levels of albumin seen at older ages are independent of nutritional status and disease.24 Albumin is also known as a negative acute phase protein, so that it can be a marker of an acute condition.25 Albumin has an essential function related to its binding property. With a low serum albumin level, the concentration of unbound drugs in the circulation is increased. This increased bioavailability of drugs may lead to adverse effects, particularly in the elderly, who have a diminished clearance. Moreover, patients with hypoalbuminemia and malnutrition are likely to have decreased immunocompetence with an increased risk of infection.23
In the current study, age has been shown to be a significant predictor of mortality in hospitalized seriously ill patients aged 65 and above. The question of whether age can predict survival is controversial. In some studies age was found as one of the main predictors of in-hospital death.26–28 However, Chelluri et al. claimed that age by itself does not appear to predict long-term survival and quality of life among elderly patients who are critically ill.29
In our patients, as in some other studies,22,30 Charlson co-morbidity index on-admission did not predict in-hospital mortality, probably because chronic co-morbidity has a lower impact on short-term mortality. Death during a hospital stay is rather influenced by acute complications or therapeutic procedures.30 Charlson co-morbidity index, though, has been found to be a predictor of mortality in a nursing home over a period of 6 months31 and also 2 years after discharge.32
As in some other studies,7–12 on-admission vitamin B12 level appears as a strong predictor of subsequent in-hospital mortality in older patients. The Bronx Aging study, a longitudinal 10-year study in community living elderly, also found an increased incidence of mortality in subjects with increased levels of vitamin B12.9 The cause of the association between vitamin B12 serum levels and mortality is unclear. Vitamin B12 level could be a marker for mortality or have some toxic effect leading to death. It has been proposed that high levels of vitamin B12 can be hepatotoxic.9 Further investigation is needed to define the mechanisms and the implications of elevated vitamin B12 levels in the geriatric population.
Elasticity calculation demonstrated that vitamin B12 was 3-fold better in predicting mortality than Charlson co-morbidity index. This finding shows that mortality is more sensitive to changes in vitamin B12 than to the changes in the Charlson co-morbidity index. Vitamin B12 has been also found by Baztan et al.12 to be a better predictor of mortality than Charlson comorbidity index. The limitation of this study lies in its retrospective design, in which the data were collected from computerized records and some data could not be attained, such as over-the-counter vitamin B12 taken at home before hospitalization and not reported on admission. But because the data on drug and supplement administration in the community clinics of the health medical organizations are available in the KMC electronic medical records, this limitation seems to be negligible. Another limitation of this study is the use of plasma vitamin B12 concentration to define vitamin B12 deficiency without metabolite (plasma homocysteine or methylmalonic acid) measurements for confirmation. There is a disagreement as to whether serum measurement of vitamin B12 accurately reflects storage levels of this vitamin. It is possible that misclassified cases and controls based on serum vitamin B12 measurements may have introduced bias, but this limitation may be considered negligible because of the large population.
Elevated vitamin B12 level was considered an important biomarker independent of age and gender to assess high risk of mortality that should be, therefore, included in severity-of-illness measures in all elderly inpatients.11 The highest mortality rate in our patients was found in the group of patients who were concomitantly in the lowest albumin quartile and the highest vitamin B12 quartile. Studies reported that high vitamin B12 levels were significantly associated with diminished albumin levels.11,33
Conclusion
In view of the fact that vitamin B12 levels have been found to predict mortaliy, they should be measured in geriatric practice, in addition to albumin levels, as a practical and reliable tool for identifying high risk elderly hospitalized patients. Probably, a combination of two or more available and inexpensive routinely taken tests can give a better estimation of mortality than some complicated tools, like Charlson co-morbidity index.
Conflict of interest: None declared.