Abstract
Background
Achievement of glycemic control is an important objective in the management of type 2 diabetes mellitus (T2DM).
Objective
The objective of this study was to evaluate the safety and efficacy of the dipeptidyl peptidase-4 inhibitor saxagliptin versus placebo as add-on therapy in patients with T2DM inadequately controlled with insulin alone or insulin plus metformin.
Methods
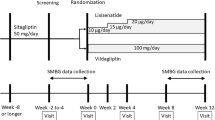
This was a long-term (28-week) extension of a short-term (24-week), randomized, double-blind, parallel-group trial of saxagliptin 5 mg once daily versus placebo as add-on therapy to open-label insulin or insulin plus metformin therapy totaling 52 weeks of treatment. In contrast with the goal of maintaining a stable insulin dosage during the short-term phase, during the extension phase the insulin dosage was flexible and adjusted as deemed appropriate by the investigator. The study was conducted in a clinical practice setting, including family practice and hospital sites. Patients with T2DM aged 18–78 years with glycated hemoglobin (HbA1c) 7.5–11 % on a stable insulin regimen (30–150 U/day with or without metformin) for ≥8 weeks at screening were included in the study. Patients were stratified by metformin use and randomly assigned 2:1 to oral saxagliptin 5 mg (n = 304) or placebo (n = 151) once daily. All patients who completed the initial 24 weeks of treatment were eligible to participate in the 28-week extension, regardless of whether they had required rescue treatment. The main outcome measure was change in HbA1c from baseline to week 52.
Results
In general, the outcomes achieved at week 24 were sustained to week 52. Adjusted mean change from baseline HbA1c at week 52 was greater with saxagliptin (−0.75 %) versus placebo (−0.38 %); the adjusted between-group difference was −0.37 % (95 % CI −0.55 to −0.19); between-group differences were similar in patients treated with metformin (−0.37 % [95 % CI −0.59 to −0.15]) and without metformin (−0.37 % [95 % CI −0.69 to −0.04]). At week 52, a greater proportion of patients receiving saxagliptin achieved HbA1c <7 % than those receiving placebo (21.3 vs. 8.7 %; between-group difference 12.6 % [95 % CI 6.1–19.1]). The increase from baseline in mean total daily insulin dose at week 52 was numerically smaller with saxagliptin (5.67 vs 6.67 U with placebo; difference, −1.01 U [95 % CI −3.24 to 1.22]). During the 52-week study period, the proportion of patients reporting ≥1 adverse event (AE) was 66.4 % with saxagliptin and 71.5 % with placebo, the majority being mild or moderate in intensity. The most common AEs (≥5 % with saxagliptin or placebo) were urinary tract infection, nasopharyngitis, upper respiratory tract infection, headache, influenza, and pain in extremity; the incidence of each AE was similar between treatment groups. In the saxagliptin and placebo groups, the incidence of reported hypoglycemia was 22.7 and 26.5 %, respectively; the incidence of confirmed hypoglycemia (fingerstick glucose ≤50 mg/dL [≤2.77 mmol/L] with characteristic symptoms) was 7.6 and 6.6 %, respectively. Adjusted mean change from baseline body weight was +0.8 kg with saxagliptin and +0.5 kg with placebo.
Conclusion
Saxagliptin 5 mg once daily as add-on to insulin, with or without concomitant metformin, produced a durable improvement in glycemic control and was well tolerated over 52 weeks of treatment.




Similar content being viewed by others
References
DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88(4):787–835, ix.
UK Prospective Diabetes Study Group. U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes. 1995;44(11):1249–58.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–79.
Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009;15(6):540–59.
Pradhan AD, Everett BM, Cook NR, et al. Effects of initiating insulin and metformin on glycemic control and inflammatory biomarkers among patients with type 2 diabetes: the LANCET randomized trial. JAMA. 2009;302(11):1186–94.
Barnett AH, Cradock S, Fisher M, et al. Key considerations around the risks and consequences of hypoglycaemia in people with type 2 diabetes. Int J Clin Pract. 2010;64(8):1121–9.
Charbonnel B, Cariou B. Pharmacological management of type 2 diabetes: the potential of incretin-based therapies. Diabetes Obes Metab. 2011;13(2):99–117.
Holman RR, Thorne KI, Farmer AJ, et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med. 2007;357(17):1716–30.
Gordon J, Pockett RD, Tetlow AP, et al. A comparison of intermediate and long-acting insulins in people with type 2 diabetes starting insulin: an observational database study. Int J Clin Pract. 2010;64(12):1609–18.
Freeman JS. Managing hyperglycemia in patients with type 2 diabetes mellitus: rationale for the use of dipeptidyl peptidase-4 inhibitors in combination with other oral antidiabetic drugs. J Am Osteopath Assoc. 2010;110(9):528–37.
Freeman JS. A physiologic and pharmacological basis for implementation of incretin hormones in the treatment of type 2 diabetes mellitus. Mayo Clin Proc. 2010;85(12 suppl):S5–14.
ONGLYZA (saxagliptin). Full prescribing information. Princeton, NJ: Bristol-Myers Squibb; 2011.
Barnett AH, Charbonnel B, Donovan M, et al. Effect of saxagliptin as add-on therapy in patients with poorly controlled type 2 diabetes on insulin alone or insulin combined with metformin. Curr Med Res Opin. 2012;28(4):513–23.
Fonseca V, Schweizer A, Albrecht D, et al. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia. 2007;50(6):1148–55.
Rosenstock J, Rendell MS, Gross JL, et al. Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA(1C) without causing weight gain or increased hypoglycaemia. Diabetes Obes Metab. 2009;11(12):1145–52.
Vilsboll T, Rosenstock J, Yki-Jarvinen H, et al. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12(2):167–77.
Ratner R, Wynne A, Nakhle S, et al. Influence of preprandial vs. postprandial insulin glulisine on weight and glycaemic control in patients initiating basal-bolus regimen for type 2 diabetes: a multicenter, randomized, parallel, open-label study (NCT00135096). Diabetes Obes Metab. 2011;13(12):1142–8.
Neumiller JJ, Campbell RK. Saxagliptin: a dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes mellitus. Am J Health Syst Pharm. 2010;67(18):1515–25.
Richter B, Bandeira-Echtler E, Bergerhoff K, et al. Emerging role of dipeptidyl peptidase-4 inhibitors in the management of type 2 diabetes. Vasc Health Risk Manag. 2008;4(4):753–68.
Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298(2):194–206.
Jabbour S, Ziring B. Advantages of extended-release metformin in patients with type 2 diabetes mellitus. Postgrad Med. 2011;123(1):15–23.
Fonseca V, Baron M, Shao Q, et al. Sustained efficacy and reduced hypoglycemia during one year of treatment with vildagliptin added to insulin in patients with type 2 diabetes mellitus. Horm Metab Res. 2008;40(6):427–30.
Acknowledgments
Drs Barnett and Charbonnel recruited and treated patients, and all authors contributed to the analysis and interpretation of study data and the preparation, review, and final approval of the manuscript. All authors confirm that this paper is an accurate representation of the study results. Bristol-Myers Squibb and AstraZeneca funded the study and participated in the study design and collection, analysis, and interpretation of the data and review of the manuscript. The decision to submit the manuscript to Clinical Drug Investigation was made independently by the authors. Medical writing support for the preparation of this manuscript was provided by Erica Wehner, RPh, and Nancy Sheridan from Complete Healthcare Communications, Inc. (Chadds Ford, PA, USA), with funding from Bristol-Myers Squibb and AstraZeneca. Karen May from Bristol-Myers Squibb contributed expertise and insights to the conduct of the study at every stage. Drs Li, Donovan, and Iqbal are employees of Bristol-Myers Squibb and have ownership of company stock. At the time of writing this manuscript, Dr Fleming was an employee of Bristol-Myers Squibb. Dr Barnett has received honoraria for lectures and advisory work as well as research funding from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Sanofi-Aventis, and Takeda. Dr Charbonnel has received fees for consultancy, speaking, travel, or accommodation from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, GlaxoSmithKline, Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Roche, Sanofi-Aventis, and Takeda.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration: ClinicalTrials.gov identifier: NCT00757588.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barnett, A.H., Charbonnel, B., Li, J. et al. Saxagliptin Add-on Therapy to Insulin With or Without Metformin for Type 2 Diabetes Mellitus: 52-Week Safety and Efficacy. Clin Drug Investig 33, 707–717 (2013). https://doi.org/10.1007/s40261-013-0107-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-013-0107-8