Abstract
The purpose of the study is to describe what is the presentation of breast cancer in women with HIV, their tolerance to therapy, the most common complications of treatment and their outcomes. Retrospective chart review of patients with HIV diagnosed with breast cancer between January 1, 1989 and December 31, 2013 at the University of Miami/Jackson Memorial Hospital (UM/JMH) 47 females and 1 male were included in the analysis. The median age of diagnosis was 46 years (IQR 41–52) and 64 % of the women were premenopausal. Median CD4+ count was 330 cells/µL (IQR 131–589 cells/µL). 41 % had AIDS at time of diagnosis. 94 % of patients presented with locoregional disease and 6 % with late stage breast cancer. 52 % had ER+ tumors. 6 % had HER-2/neu tumor expression and 21 % had triple negative disease. The 5 year PFS was 50 % (95 % CI 34–64 %), the 5 year OS was 44 % (95 % CI 29–58 %), and the Breast cancer-specific survival was 57 % (95 % CI 40–70 %). Death was attributed to breast cancer in 22 patients, AIDS progression in 6 patients, other medical condition in 1, and for 4, the cause was unknown. Serious adverse events were documented in 46 % of patients treated with chemotherapy. Targeted therapy was well tolerated. Patients with HIV/AIDS and breast cancer pose a major challenge for oncologists. Surgery, radiation, and endocrine therapy are well tolerated. Standard dose chemotherapy can have life-threatening side effects which can be managed with growth factor support and antimicrobial prophylaxis. All cancer therapy can be given while continuing with antiviral therapy at full dose.
Similar content being viewed by others
Introduction
As the prevalence of women living with HIV rises, increase attention is needed to the long-term health maintenance and care in this specific population. In the general population, breast cancer is the most common malignancy in women (excluding skin cancer) in the US. and the second most common cause of cancer-related death in women with an estimate of 232,670 new cases to be diagnosed in 2014, causing about 40,000 deaths each year from the disease [1–3]. It is well established that since the introduction of highly active antiretroviral therapy (HAART), the life expectancy of HIV-infected individuals has increased, while the incidence of both opportunistic infections and AIDS-defining cancers (ADCs) has dramatically decreased [4, 5] With this shift, the rate of Non AIDS-Defining Cancers (NADCs) has risen and now accounts for the majority of cancers in HIV-infected persons [6]. For breast cancer, however, initial reports documented a decrease incidence in cohorts from HIV patients relative to the general population [7–10]. Later studies have suggested that the risk has increased significantly in the last years and is now approaching the risk of the general population [11–16]. According to the latest report of the World Health Organization [17], the proportion of women living with HIV has been increasing in the last 10 years and now constitutes 50 % of people living with HIV worldwide, the cases of breast cancer in this population are therefore expected to increase.
Treating patients with HIV and breast cancer remain a challenge. In 2001, we reported our experience with 20 patients with breast cancer and HIV and it was the largest cohort to that date [18]. Since then, even though there have been continuous reports, it still remains the report of small case series and a few series linking cancer to HIV registries [9, 11, 13, 15, 19–25]. The recent report of 151 patients in Soweto, Africa [12] constitutes the largest cohort to date and they reported equal prevalence to the HIV-negative cohort; however, no information regarding treatment and outcome was given. There are currently no approved guidelines or recommendations for screening or for treating HIV patients with breast cancer and clinicians must make decisions despite the lack of well-validated data regarding outcomes, tolerability, safety, interactions, and management of predictive clinical variables unique to HIV patients like CD4 and viral load [11, 26].
We therefore aim to present an updated report of our experience with 48 cases of HIV-seropositive women with carcinoma of the breast diagnosed between January 1, 1989 and December 31, 2013 in order to share the key points learned regarding presentation, choice of therapy, and expected complications and outcomes.
Patients and methods
Study population and study design
This study was approved by the University of Miami IRB. This was a retrospective registry-based analysis that included all HIV-positive patients who received their breast cancer care at the University of Miami/Jackson Memorial Hospital (UM/JMH) between January 1, 1989 and December 31, 2013. Cases were identified by a review of the JMH Oncology Clinic patient roster and the breast cancer clinic patient registry from Sylvester Comprehensive Cancer Center (SCCC). All cases were confirmed by chart review. HIV testing was not routinely performed. HIV infection was determined by enzyme-linked immunosorbent assay (ELISA), with confirmation by Western blot analysis. Breast cancer diagnoses were confirmed by histology. Cases in which HIV status had been diagnosed after initial treatment were excluded from the analysis.
Definitions and endpoints
Data were collected on patient demographics, HIV, disease and treatment characteristics, and clinical outcome. Demographic variables included age at diagnosis and race/ethnicity. HIV-related variables included date of diagnosis, CD4+ count, and viral load (VL) at breast cancer diagnosis, risk factors for HIV (Intravenous drug abuse, unprotected sexual intercourse, and Blood transfusion) and history of any AIDS-defining condition (ADC). AIDS was defined by CDC case definition as a CD4 count < 200 or any history of ADC. HAART therapy at diagnosis was established.
Cancer-related variables included T, N, M, stage, Estrogen (ER), and Progesterone (PR) receptor expression evaluated by immunohistochemistry and Her-2/neu expression evaluated by either fluorescence in situ hybridization (FISH), Chromogenic in situ hybridization (CISH) or IHC. Type of chemotherapy, targeted therapy (either hormonal or Her-2 directed), and radiation was recorded for each patient. Progression-free survival (PFS) was defined as the time from cancer diagnosis to relapse, disease progression, or death from any cause. Overall survival (OS) was defined as the time from cancer diagnosis to death from any cause. Death was confirmed by clinical records, the Social Security Death Index, or family member, and cause of death was classified as related to breast cancer, AIDS, other clinical conditions and unknown. Breast cancer-specific survival was defined as the time from diagnosis to the time of a breast cancer-related death.
Statistical analysis
Patient, HIV- and cancer-related variables were described as categorical, discrete, or continuous variables as appropriate with corresponding measurements of central tendencies, absolute and relative frequencies, and dispersion measures (Interquartile range (ICR) for non-normal data and standard deviation (SD) for normal data). Discrete estimates were rounded to the closest whole number. We calculated 95 % confidence intervals (CI) or standard error (SE) where appropriate. The probabilities of PFS, OS, and Breast cancer-specific survival were estimated from the time of Breast cancer diagnosis using Kaplan–Meier survival curves and calculating their confidence interval. Univariate analyses of the effect of stage on PFS, OS, and breast cancer-specific survival were analyzed by Cox proportional hazard ratios (HR); Multivariable analysis was not pursued due to the small cohort. Data were analyzed by complete case analysis and no imputation data were used for missing data. Stata 12.0 was used for statistical analysis.
Results
A total of 55 possible cases were identified by review of the JMH Oncology Clinic patient roster and the breast cancer clinic patient registry from SCCC between January 1, 1988 and December 31, 2013, and were included for chart review. Of these, 2 were excluded because they were HIV-negative patients, 3 because HIV was diagnosed after treatment, and 2 because chart was unavailable for review. A total of 48 cases were included in the analysis.
Baseline patient characteristics
Demographic, HIV- and cancer-related variables are summarized in Table 1.
Demographics
There were 47 female patients and 1 male patient with breast cancer and HIV infection. The median age of breast cancer diagnosis was 46 years (IQR 41–52). Thirty females (64 %) were premenopausal. 65 % were African American, 19 % Hispanic, 10 % Caribbean, and 6 % were Caucasian.
HIV related
The initial date of diagnosis of HIV infection was available for 30 patients (63 %). Median time of HIV diagnosis to Breast Cancer diagnosis was 4 years (ICR 1–11 years). A CD4+ count in patients with HIV/AIDS at the time of initial breast cancer diagnosis was available in 39 patients. Median CD4+ count was 330 cells/µL (IQR 131–589 cells/µL). A viral load was available in 16 patients at time of diagnosis: an undetectable viral load in 6 patients and in the other 10 ranged between 949 and 299,000 copies/µL. 20 patients (41 %) had documented AIDS at time of diagnosis of breast cancer; 15 with CD4 count below 200 cells/µL ± ADC and 5 additional had a CD4+ count > 200 cells/µL with a documented history of ADC (Pneumocystis jirovecii pneumonia in 2, Cytomegalovirus retinitis, Toxoplasmosis of brain, and esophageal candidiasis in one patient each). Risk factors for HIV were not reported in half of the charts reviewed and HAART was only reported in 20 patients at diagnosis. Of these, only 12 were on active HAART treatment on diagnosis.
Cancer related
Two percent of patient had Stage 0 disease, 8 % Stage I, 44 % Stage II, 40 % Stage III, and 6 % had stage IV. The median tumor size was 3.6 cm (IQR 2.8–6.7 cm), and clinical or pathological lymph node involvement was presented in 31 patients (65 %).
Treatment
Discrimination of treatment by stage is shown in Table 2.
Locoregional disease (n = 45)
For the 45 patients who presented with non-metastatic disease, 14 underwent neoadjuvant chemotherapy (31 %) and one patient had progressive disease during neoadjuvant treatment. Definitive surgery was recommended for 44 patients but 3 patients declined. In the other 41 patients who consented to surgery, modified radical mastectomy or breast conservative therapy (BCT) was performed in 31 and 9 patients, respectively. One patient opted for bilateral mastectomy.
Adjuvant treatment included chemotherapy in 18 (40 %), hormonal therapy alone in 8 patients (18 %) and in combination with chemotherapy in 13 (29 %), Her-2 directed in 1 with chemotherapy and radiation (XRT) in 14 patients (32 %). Seven patients refused either chemotherapy or radiation. Of the 29 patients with stages I–III disease, who had received chemotherapy as neoadjuvant or adjuvant, 24 received an anthracycline-based chemotherapy.
Late stage breast cancer (n = 3)
Three patients presented with stage IV disease at the time of initial breast cancer diagnosis and all were ER+. 1 patient received anthracycline-based chemotherapy and died from neutropenic fever during the first cycle, the other 2 received endocrine therapy alone.
Hormonal and Her-2 over expression
ER, PR, and Her-2 status were available for 44, 35, and 27 patients, respectively. 25 tumors (52 %) were known to have positive hormonal receptor. 10 patients received solely endocrine therapy and 13 patients received it concomitantly with chemotherapy at some point of their treatment. Of the 3 patients that were positive for Her- 2/neu over expression, 2 had concomitant ER+ expression, 1 received treatment with trastuzumab as adjuvant therapy, and the other one only received Tamoxifen. The third patient initially refused but upon relapsed, received trastuzumab, and then lapatinib was added at progression. The triple negative phenotype, i.e., ER−/PR−/HER2− was present in 10 patients who had reports of all markers.
Adverse events
All patients tolerated surgery well. From the 30 patients that receive chemotherapy either in neoadjuvant only (12), adjuvant only (14), or both (2), serious adverse events were documented in 14 patients (46 %). The most common complication was myelosuppression/neutropenic fever in 10 patients, including one death due to neutropenic fever. Two women developed Pneumocystis Carinii pneumonia during treatment. One patient had severe erosive candida esophagitis and another patient developed adult respiratory distress syndrome (ARDS) during treatment. These two patients developed acceleration of HIV disease to AIDS and death within 6 months of completing chemotherapy. Zoster infection was reported in 3 patients. One patient had elevated liver enzymes during treatment. Patients that were treated with endocrine therapy had no significant side effects. Overall, radiation therapy was well tolerated and there were no significant side effects documented.
Clinical outcomes
PFS
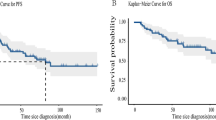
17 patients (35 %) relapsed and 8 more had progressive disease (17 %). The median PFS was 45 months, SE 36 (Fig. 1). The estimate PFS was 53 % at 3 years (95 % CI 36–67 %), 50 % at 5 years 95 % CI 34–64 %), and 40 % at 10 years (95 % CI 23–57 %). Of the 25 patients who had progressive disease or relapse, 5 (21 %) received chemotherapy, 8 (33 %) radiation, and 5 (21 %) received targeted therapy. Treatment for PD or Relapse by stage is also provided in Table 2. The PFS was associated with initial stage (Fig. 2), HR 3.59 (p < 0.000). 5 year PFS by Stage was 100 % for Stage I, 56 % for Stage II (95 % CI 31–75 %), 38 % for Stage III (95 % CI 16–60 %), and 0 % for Stage IV.
OS
The median length of follow-up was 42 months (range from 1 to 243 months). Thirty-three patients (69 %) died during follow-up. The most common cause of death was breast cancer in 22 patients out of the 25 patients who had PD or relapsed. One of the patients is still following in clinic and the other two patients were lost to follow-up after disease progression. Six patients died from AIDS-related complications. One patient died from other medical condition and for four, the cause of death was unknown and death was confirmed by social security data. The median OS after breast cancer diagnosis was 52 months, SE 9 (Fig. 3). The estimate OS was 61 % at 3 years (95 % CI 46–73 %), 44 % at 5 years (95 % CI 29–58 %), and 28 % at 10 years (95 % CI 15–43 %).
Breast cancer-specific survival
The median breast cancer-specific survival was 117 months, SE 45. The estimate breast cancer-specific survival was 68 % at 3 years (95 % CI 52–80 %), 57 % at 5 years (95 % CI 40–70 %), and 47 % at 10 years (95 % CI 30–64 %). Breast cancer-specific OS was also associated with initial stage (Fig. 4), HR 3.00 (p < 0.001). 5 year Breast cancer-specific survival by Stage (Fig. 4) was 100 % for Stage I, 66 % for Stage II (95 % CI 39–83 %), 43 % for Stage III (95 % CI 17–67 %), and 0 % for Stage IV. The only patient with DCIS died from AIDS progression, and 5 year survival was unavailable.
Discussion
Comparing our 48 consecutive patients with HIV and breast cancer treated in a single institution to the historical data for the general breast cancer population, we found that our cohort was more likely to be young, and African American. The median age was 46 at the time of breast cancer diagnosis compared to 61 for the general population in the period 2006–2010. Our patients were younger than the median age for African Americans women without breast cancer (57 years) [3]. Only 33 % of our patients were older than 50 years compared to 79 % in the general population [27]. The occurrence of breast cancer in younger patients with HIV has been a common finding between reported case series [11–13, 19]; however, a recent study reported that these differences disappear after adjusting for differences in the populations at risk of HIV patients [28]. Half of our patients had ER-positive breast cancer and 6 % were HER-2 overexpressing.
Breast cancer was the main cause of death for the cohort and as anticipated, mortality of breast cancer was statistically related to the stage of the disease at presentation. In order to provide a context for our results, the overall survival of this cohort of HIV-infected women with breast cancer had higher breast cancer-specific mortality compared to the general American population and to the African American population. Breast cancer-specific OS at 5 years was 57 % in our HIV women compared to 89.2 % for general population reported by the SEER data base for the 2004–2010 period [3]. Caution should be used when interpreting these long-term survival rates because they represent patients who were diagnosed over the course of 15 years and therapy for both breast cancer and HIV has improved dramatically over this time period. [2]. In addition, these comparisons should be regarded with uncertainty as no direct assessment can be made between retrospective data with historical statistics as many things can be influencing the results, and no conclusion can be reached regarding behavior of breast cancer in HIV patients.
The competing risk and effect of HIV/AIDS in presentation, treatment complications and mortality are of importance interest in this population. Our cohort had similar distribution of CD4 count categories as the general population of women with HIV infection. There has been no association between HIV or CD4 count and stage at diagnosis for breast cancer patients [12, 15, 16]. In our series most women presented with stage II and III (83 %), which is higher than for the general population but more in line with what is seen in our safety net clinic population where the combined rate of Stage 2 and 3 is approximately 65 %.
The rationale for choosing specific therapeutic regimens in the HIV population has not been codified. The incidence of myelosuppression with chemotherapy appears to be higher in the HIV-infected population based on our initial report [18] where 5 out of 7 patients developed myelosuppression, and these findings have also been reported in others experience [20, 29]. In our updated and larger cohort, the incidence of serious adverse events was documented in 14 out of 30 patients that receive chemotherapy which reflects a proportionally decrease in the rate of complications. In addition to myelosuppression, zoster was a common complication as was thrush and vaginal candidiasis. In an attempt to minimize chemotherapy-related complication, we have adopted a number of strategies. We use weekly therapy rather than every 3 week therapy and we never use dose dense regimens since Burstein reported the occurrence of pneumocystisis carinii pneumonia in 2 women without HIV infection treated with dose dense AC-T [30]. We use ovarian suppression plus tamoxifen in women whose tumors are estrogen receptor positive instead of chemotherapy. We avoid steroid premedication when possible and we substitute Nab-Paclitaxel for Paclitaxel on occasion to avoid weekly steroid administration. We use growth factor support liberally and give prophylactic fluconazole, trimethoprim/sulfamethoxazole, and acyclovir to most women receiving chemotherapy. In patients with space occupying lesions of the brain we initiate therapy for toxoplasmosis. If there is no response to therapy for toxoplasmosis and the csf shows no Epstein Barr Virus (EBV) infection we consider that the patient has metastatic breast cancer to the brain and treat accordingly.
Drug interaction between chemotherapy and HAART is still an area of concern but limited data exist with breast cancer-specific regimens [26]. The earlier patients in our study did not receive HAART therapy and they had many more complications than the later patients in the study. We did not stop or modify HAART therapy for the patients receiving therapy. In the Soweto report [12], the experience of treating patients with very low CD4 count presented especial problems, as aggressive chemotherapy given without HAART could be rapidly fatal and holding chemotherapy until increased CD4 was not an option. They recommended initiating chemotherapy after initiating ART; however, no data were reported on complication, thus we cannot make any conclusions about the relationship between HAART and treatment on our patients
Our specific population may limit the external validity of our results. In 2010, 94,897 people were living with a diagnoses of HIV in Florida (a state with one of highest prevalence), of these 46, 248 (48 %) were African American despite making up only 16 % of the total population [31]. In Miami-Dade County, African American compromise 50 % of total HIV/AIDS population but represent 20 % of the general population [32]. These numbers are even starker in black women, where the black female to white female ratio of HIV/AIDS cases is 10 to 1 in Miami-Dade County.
The intrinsic worse prognosis of breast cancer in women of African descent where incidence is higher in younger women who have higher grade and hormone receptor-negative tumors compared to than those diagnosed in white women [33–35] is compounded by lack of access to health care. The fact that most of the women with breast cancer and HIV are of African descent may complicate the question of what role the HIV infection has on the innate lethality of the disease.
The main strength of this series is that it tracks the long-term experience of a single institution in the treatment of breast cancer in patients with HIV/AIDS. It provides useful although anecdotal experience on treatment strategies that have been developed over many years. With significant limitation in available cases even in a referral center as ours, observational studies constitute the best available data.
There are still many unanswered questions. Further studies to confirm the association between HIV status and survival with cancer, role, and limitations of HAART within selection of therapy, and optimal standard of care for this specific population persists as interesting areas of research. Both screening and adjuvant treatment have been associated with reduction of death from breast cancer in the general population, [1]; the application to this HIV subgroup remains to be optimized.
In conclusion, we have demonstrated in this updated series that treating breast cancer in HIV-infected woman is possible but remains a challenge to the oncologist. Surgery and endocrine-directed therapy remain well tolerated but standard-dose chemotherapy can have life-threatening side effects in HIV-infected patients. Hence, the benefits of adjuvant chemotherapy in this population have to be carefully weighed against the high incidence of complications and adequate choice of the least myelotoxic regimen. From our experience, if given with adequate growth factor support and prophylaxis, it can potentially improve outcomes. With increase life expectancy related to HIV with adequate HAART, the main cause of death in this women is expected be related to the breast cancer or treatment and adequate management will be key to survival but further research efforts are needed in this area.
References
Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema JD, Feuer EJ (2005) Effect of screening and adjuvant therapy on mortality from breast cancer. New Engl J Med 353(17):1784–1792. doi:10.1056/NEJMoa050518
American Cancer Society (2014) Cancer facts and figures 2014. American Cancer Society, Atlanta
Surveillance, Epidemiology, and End Results Program (SEER); Stat Fact Sheets: Breast Cancer (SEER Cancer Statistics Review, 1975–2010.) National Cancer Institute. http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission
Crum-Cianflone N, Hullsiek KH, Marconi V, Weintrob A, Ganesan A, Barthel RV, Fraser S, Agan BK, Wegner S (2009) Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS (London, England) 23(1):41–50. doi:10.1097/QAD.0b013e328317cc2d
Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD (1998) Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV outpatient study investigators. N Engl J Med 338(13):853–860. doi:10.1056/nejm199803263381301
Powles T, Robinson D, Stebbing J, Shamash J, Nelson M, Gazzard B, Mandelia S, Moller H, Bower M (2009) Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J Clin Oncol 27(6):884–890. doi:10.1200/jco.2008.19.6626
Frisch M, Biggar RJ, Engels EA, Goedert JJ (2001) Association of cancer with AIDS-related immunosuppression in adults. JAMA, J Am Med Assoc 285(13):1736–1745
Amir H, Kaaya EE, Kwesigabo G, Kiitinya JN (2000) Breast cancer before and during the AIDS epidemic in women and men: a study of Tanzanian Cancer Registry Data 1968 to 1996. J Natl Med Assoc 92(6):301–305
Pantanowitz L, Dezube BJ (2001) Breast cancer in women with HIV/AIDS. JAMA, J Am Med Assoc 285(24):3090–3091
Hessol NA, Napolitano LA, Smith D, Lie Y, Levine A, Young M, Cohen M, Minkoff H, Anastos K, D’Souza G, Greenblatt RM, Goedert JJ (2010) HIV tropism and decreased risk of breast cancer. PLoS ONE 5(12):e14349. doi:10.1371/journal.pone.0014349
Spano JP, Lanoy E, Mounier N, Katlama C, Costagliola D, Heard I (2012) Breast cancer among HIV infected individuals from the ONCOVIH study, in France: therapeutic implications. Eur J Cancer 48(18):3335–3341. doi:10.1016/j.ejca.2012.05.019
Cubasch H, Joffe M, Hanisch R, Schuz J, Neugut AI, Karstaedt A, Broeze N, van den Berg E, McCormack V, Jacobson JS (2013) Breast cancer characteristics and HIV among 1,092 women in Soweto, South Africa. Breast Cancer Res Treat 140(1):177–186. doi:10.1007/s10549-013-2606-y
Andrade AC, Luz PM, Veloso VG, Cardoso SW, Moreira RI, Grinsztejn B, Friedman RK (2011) Breast cancer in a cohort of human immunodeficiency virus (HIV)-infected women from Rio de Janeiro, Brazil: a cases series report and an incidence rate estimate. Braz J Infect Dis 15(4):387–393
Goedert JJ, Schairer C, McNeel TS, Hessol NA, Rabkin CS, Engels EA (2006) Risk of breast, ovary, and uterine corpus cancers among 85,268 women with AIDS. Br J Cancer 95(5):642–648. doi:10.1038/sj.bjc.6603282
Sarhan M, DePaz HA, Oluwole SF (2010) Breast cancer in women with human immunodeficiency virus infection: pathological, clinical, and prognostic implications. J Women’s Health 19(12):2261–2266. doi:10.1089/jwh.2010.2026
Shaaban HS, Modi Y, Guron G (2012) Is there an association between human immunodeficiency virus infection and breast cancer? Med Oncol 29(2):446–447. doi:10.1007/s12032-011-9856-5
Health DoGWa, World Health Organization (2009) Integrating gender into HIV/AIDS programmes in the health sector: tool to improve responsiveness to women’s needs. Bulletin of the World Health Organization. World Health Organization, Geneva
Hurley J, Franco S, Gomez-Fernandez C, Reis I, Velez P, Doliny P, Harrington W Jr, Wilkinson J, Kanhoush R, Lee Y (2001) Breast cancer and human immunodeficiency virus: a report of 20 cases. Clin Breast Cancer 2(3):215–220. doi:10.3816/CBC.2001.n.024 (discussion 221)
Calabresi A, Ferraresi A, Vavassori A, Castelli F, Quiros-Roldan E (2012) Breast cancer among human immunodeficiency virus (HIV)-infected patients: the experience in Brescia, Northern Italy. Braz J Infect Dis 16(4):396–397. doi:10.1016/j.bjid.2012.06.001
El-Rayes BF, Berenji K, Schuman P, Philip PA (2002) Breast cancer in women with human immunodeficiency virus infection: implications for diagnosis and therapy. Breast Cancer Res Treat 76(2):111–116
Guth AA (2003) Breast cancer and human immunodeficiency virus infection: issues for the 21st century. J Women’s Health 12(3):227–232. doi:10.1089/154099903321667564
Intra M, Gentilini O, Brenelli F, Chagas EM, Veronesi U, Sandri MT (2005) Breast cancer among HIV-infected patients: the experience of the European Institute of Oncology. J Surg Oncol 91(2):141–142. doi:10.1002/jso.20315
Latif N, Rana F, Guthrie T (2011) Breast cancer and HIV in the era of highly active antiretroviral therapy: two case reports and review of the literature. Breast J 17(1):87–92. doi:10.1111/j.1524-4741.2010.01023.x
Oluwole SF, Ali AO, Shafaee Z, DePaz HA (2005) Breast cancer in women with HIV/AIDS: report of five cases with a review of the literature. J Surg Oncol 89(1):23–27. doi:10.1002/jso.20171
Petoumenos K, Hui E, Kumarasamy N, Kerr SJ, Choi JY, Chen YM, Merati T, Zhang F, Lim PL, Sungkanuparph S, Pujari S, Ponnampalavanar S, Ditangco R, Lee CK, Grulich A, Law MG (2010) Cancers in the TREAT Asia HIV Observational Database (TAHOD): a retrospective analysis of risk factors. J Int AIDS Soc 13:51. doi:10.1186/1758-2652-13-51
Mounier N, Katlama C, Costagliola D, Chichmanian RM, Spano JP (2009) Drug interactions between antineoplastic and antiretroviral therapies: implications and management for clinical practice. Crit Rev Oncol/Hematol 72(1):10–20. doi:10.1016/j.critrevonc.2008.10.013
American Cancer Society (2013) Breast cancer facts and figures 2013–2014. Ame Cancer Soc Inc, Atlanta
Shiels MS, Pfeiffer RM, Engels EA (2010) Age at cancer diagnosis among persons with AIDS in the United States. Ann Intern Med 153(7):452–460. doi:10.7326/0003-4819-153-7-201010050-00008
Garcia-Tejedor A, Devesa NR, Suarez-Pumariega P, del Barco S, Huerta MV (2007) Breast cancer and HIV–the adverse effects chemotherapy. Breast J 13(6):622–623. doi:10.1111/j.1524-4741.2007.00498.x
Tolaney SM, Najita J, Winer EP, Burstein HJ (2008) Lymphopenia associated with adjuvant anthracycline/taxane regimens. Clin Breast Cancer 8(4):352–356. doi:10.3816/CBC.2008.n.041
NCHHSTP Atlas. (2010) CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (NCHHSTP). http://gis.cdc.gov/GRASP/NCHHSTPAtlas/main.html. Accessed 20 May 2014
Number of Reported AIDS and HIV cases in 2011, 2012 and 2013 http://www.dadehealth.org/downloads/Reported%20AIDS%20&%20HIV%20cases%202011-2013.pdf. Accessed 20 May 2014
American Cancer Society (2013) Cancer facts and figures for African Americans 2013–2014. American Cancer Society
Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC (2006) Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA, J Am Med Assoc 295(21):2492–2502. doi:10.1001/jama.295.21.2492
Li CI, Malone KE, Daling JR (2002) Differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older. Cancer Epidemiol biomark Prev 11(7):601–607
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This research project complies with the current laws of the US.
Funding sources
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gomez, A., Montero, A.J. & Hurley, J. Clinical outcomes in breast cancer patients with HIV/AIDS: a retrospective study. Breast Cancer Res Treat 149, 781–788 (2015). https://doi.org/10.1007/s10549-015-3275-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3275-9