Abstract
Electronic health records (EHRs) provide opportunities to enhance patient care, embed performance measures in clinical practice, and facilitate clinical research. Concerns have been raised about the increasing recruitment challenges in trials, burdensome and obtrusive data collection, and uncertain generalizability of the results. Leveraging electronic health records to counterbalance these trends is an area of intense interest. The initial applications of electronic health records, as the primary data source is envisioned for observational studies, embedded pragmatic or post-marketing registry-based randomized studies, or comparative effectiveness studies. Advancing this approach to randomized clinical trials, electronic health records may potentially be used to assess study feasibility, to facilitate patient recruitment, and streamline data collection at baseline and follow-up. Ensuring data security and privacy, overcoming the challenges associated with linking diverse systems and maintaining infrastructure for repeat use of high quality data, are some of the challenges associated with using electronic health records in clinical research. Collaboration between academia, industry, regulatory bodies, policy makers, patients, and electronic health record vendors is critical for the greater use of electronic health records in clinical research. This manuscript identifies the key steps required to advance the role of electronic health records in cardiovascular clinical research.
Similar content being viewed by others
Introduction
Electronic health records (EHRs) provide opportunities to enhance patient care, to embed performance measures in clinical practice, and to improve the identification and recruitment of eligible patients and healthcare providers in clinical research. On a macroeconomic scale, EHRs (by enabling pragmatic clinical trials) may assist in the assessment of whether new treatments or innovation in healthcare delivery result in improved outcomes or healthcare savings.
Concerns have been raised about the current state of cardiovascular clinical research: the increasing recruitment challenges; burdensome data collection; and uncertain generalizability to clinical practice [1]. These factors add to the increasing costs of clinical research [2] and are thought to contribute to declining investment in the field [1].
The Cardiovascular Round Table (CRT) of the European Society of Cardiology (ESC) convened a two-day workshop among international experts in cardiovascular clinical research and health informatics to explore how EHRs could advance cardiovascular clinical research. This paper summarizes the key insights and discussions from the workshop, acknowledges the barriers to EHR implementation in clinical research, and identifies practical solutions for engaging stakeholders (i.e., academia, industry, regulatory bodies, policy makers, patients, and EHR vendors) in the implementation of EHRs in clinical research.
Overview of electronic health records
Broadly defined, EHRs represent longitudinal data (in electronic format) that are collected during routine delivery of health care [3]. EHRs generally contain demographic, vital statistics, administrative, claims (medical and pharmacy), clinical, and patient-centered (e.g., originating from health-related quality-of-life instruments, home-monitoring devices, and frailty or caregiver assessments) data. The scope of an EHR varies widely across the world. Systems originating primarily as billing systems were not designed to support clinical work flow. Moving forward, EHR should be designed to optimize diagnosis and clinical care, which will enhance their relevance for clinical research. The EHR may reflect single components of care (e.g., primary care, emergency department, and intensive care unit) or data from an integrated hospital-wide or inter-hospital linked system [4]. EHRs may also change over time, reflecting evolving technology capabilities or external influences (e.g., changes in type of data collected related to coding or reimbursement practices).
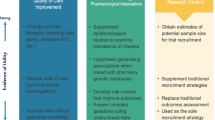
EHRs emerged largely as a means to improve healthcare quality [5–7] and to capture billing data. EHRs may potentially be used to assess study feasibility, facilitate patient recruitment, streamline data collection, or conduct entirely EHR-based observational, embedded pragmatic, or post-marketing randomized registry studies, or comparative effectiveness studies. The various applications of EHRs for observational studies, safety surveillance, clinical research, and regulatory purposes are shown in Table 1 [3, 8–10].
Electronic health records for research applications
Epidemiologic and observational research
EHR data have been used to support observational studies, either as stand-alone data or following linkage to primary research data or other administrative data sets [3, 11–14]. For example, the initial Euro Heart Survey [15] and subsequent Eurobservational Research Program (EORP) [16], the American College of Cardiology National Cardiovascular Data Registry (ACC-NCDR) [14], National Registry of Myocardial Infarction (NRMI), and American Heart Association Get With the Guidelines (AHA GWTG) [17] represent clinical data (collected from health records into an electronic case report form [eCRF] designed for the specific registry) on the management of patients across a spectrum of different cardiovascular diseases. However, modern EHR systems can minimize or eliminate the need for duplicate data collection (i.e., in a separate registry-specific eCRF), are capable of integrating large amounts of medical information accumulated throughout the patient’s life, enabling longitudinal study of diseases using the existing informatics infrastructure [18]. For example, EHR systems increasingly house imaging data which provide more detailed disease characterization than previously available in most observational data sets. In some countries (e.g., Farr Institute in Scotland [19]), the EHR can be linked, at an individual level, to other data sets, including general population health and lifestyle surveys, disease registries, and data collected by other sectors (e.g., education, housing, social care, and criminal justice). EHR data support a wide range of epidemiological research on the natural history of disease, drug utilization, and safety, as well as health services research.
Safety surveillance and regulatory uses
Active post-marketing safety surveillance and signal detection are important, emerging applications for EHRs, because they can provide realistic rates of events (unlike spontaneous event reports) and information on real-world use of drugs [20]. The EU-ADR project linked 8 databases in four European countries (Denmark, Italy, The Netherlands, United Kingdom) to enable analysis of select target adverse drug events [21]. The European Medicines Agency (EMA) coordinates the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) which aims to conduct post-marketing risk assessment using various EHR sources [22, 23]. In the United States, the Food and Drug Administration (FDA) uses EHR data from several different sources (e.g., Sentinel and Mini-Sentinel System [24], Centers for Medicare and Medicaid Services [CMS], Veterans Affairs, Department of Defense, Substance Abuse and Mental Health Services Administration) to support post-marketing safety investigations [25].
Prospective clinical research
National patient registries that contain data extracted from the EHR are an accepted modality to assess guideline adherence and the effectiveness of performance improvement initiatives [26–33]. However, the use of EHRs for prospective clinical research is still limited, despite the fact that data collected for routine medical care overlap considerably with data collected for research. The most straightforward and generally accepted application for EHR is assessing trial feasibility and facilitating patient recruitment, and EHRs are currently used for this purpose in some centers. Using EHR technology to generate lists of patients who might be eligible for research is recognized as an option to meet meaningful use standards for EHR in the United States [6]. However, incomplete data may prohibit screening for the complete list of eligibility criteria [34], but EHRs may facilitate pre-screening of patients by age, gender, and diagnosis, particularly for exclusion of ineligible patients, and reduce the overall screening burden in clinical trials [35]. A second, and more complex, step involves the reuse of information collected in EHRs for routine clinical care as source data for research. Using EHRs as the source for demographic information, co-morbidities, and concomitant medications has several advantages over separately recording these data into an eCRF. Transcription errors may be reduced, since EHR data are entered by providers directly involved in a patient’s care as opposed to secondary eCRF entry by study personnel. The eCRF may be a redundant and costly step in a clinical trial, since local health records (electronic or paper) are used to verify source data entered into the eCRF. Finally, EHRs might enhance patient safety and reduce timelines if real-time EHR systems are used in clinical trials, in contrast to delays encountered with manual data entry into an eCRF. The EHR may facilitate implementation of remote data monitoring, which has the potential to greatly reduce clinical trial costs. The Innovative Medicine Initiative (IMI) Electronic Health Records for Clinical Research (EHR4CR, http://www.ehr4cr.eu) project is one example, where tools and processes are being developed to facilitate reuse of EHR data for clinical research purposes. Systems to assess protocol feasibility and identify eligible patients for recruitment have been implemented, and efforts to link EHRs with clinical research electronic data collection are ongoing [36].
A shift towards pragmatic trials has been proposed as a mechanism to improve clinical trial efficiency [37]. Most of the data in a pragmatic trial are collected in the context of routine clinical care, which reduce trial-specific clinic visits and assessments, and should also reduce costs [38]. This concept is being applied in the National Institutes of Health (NIH) Health Care Systems Research Collaboratory. Trials conducted within the NIH Collaboratory aim to answer questions related to care delivery and the EHR contains relevant data for this purpose. Studies may have additional data collection modules if variables not routinely captured in the EHR are needed for a specific study. Similarly, the Patient-Centered Outcomes Research Institute (PCORI) has launched PCORnet, a research network that uses a common data platform alongside the existing EHR to conduct observational and interventional comparative effectiveness research [9, 39, 40].
The integration of EHRs in the conventional randomized controlled trials intended to support a new indication is more complex. EHRs may be an alternative to eCRFs when data collection is focused and limited to critical variables that are consistently collected in routine clinical care. Regulatory feedback indicates that while a new indication for a marketed drug might be achieved through EHRs, first marketing authorization using data entirely from EHRs would most likely not be possible with current systems until validation studies are performed and reviewed by regulatory agencies. The EHR could also be used to collect serious adverse events (SAE) that result in hospitalization, or to collect endpoints that do not necessarily require blinded adjudication (e.g., death), although the utility of EHRs for this purpose is dependent on the type of endpoint, whether it can reliably be identified in the EHR, and the timeliness of EHR data availability. Events that are coded for reimbursement (e.g., hospitalizations, MI) or new diagnoses, where disease-specific therapy is initiated (e.g., initiation of glucose lowering drugs to define new onset diabetes) tend to be more reliable. The reliability of endpoint collection varies by region and depends on the extent of linkage between different databases.
Challenges to using electronic health records in clinical trials and steps toward solutions
Challenges to using EHRs in clinical trials have been identified, related to data quality and validation, complete data capture, heterogeneity between systems, and developing a working knowledge across systems (Table 2). Ongoing projects, such as those conducted within the NIH Collaboratory and PCORnet [39, 41] in the United States or the Farr Institute of Health Informatics Research in Scotland, have demonstrated the feasibility of using EHRs for aspects of clinical research, particularly comparative effectiveness. The success of these endeavors is connected to careful planning by a multi-stakeholder group committed to patient privacy, data security, fair governance, robust data infrastructure, and quality science from the outset. The next hurdle is to adapt the accrued knowledge for application to a broader base of clinical trials.
Data quality and validation
Data quality and validation are key factors in determining whether EHRs might be suitable data sources in clinical trials. Concerns about coding inaccuracies or bias introduced by selection of codes driven by billing incentives rather than clinical care may be diminished when healthcare providers enter data directly into the EHRs or when EHRs are used throughout all areas of the health-system, but such systems have not yet been widely implemented [42]. Excessive or busy workloads may also contribute to errors in clinician data entry [43]. Indeed, errors in EHRs have been reported [43–45].
Complete data capture is also a critical aspect of using EHRs for clinical research, particularly if EHRs are used for endpoint ascertainment or SAE collection. Complete data capture can be a major barrier in regions, where patients receive care from different providers or hospitals operating in different EHR systems that are not linked.
Consistent, validated methods for assessing data quality and completeness have not yet been adopted [46], but validation is a critical factor for the regulatory acceptance of EHR data. Proposed validation approaches include using both an eCRF and EHRs in a study in parallel and comparing results using the two data collection methods. This approach will require collaborative efforts to embed EHR substudies in large cardiovascular studies conducted by several sponsors. Assessing selected outcomes of interest from several EHR-based trials to compare different methodologies with an agreed statistical framework will be required to gauge precision of data collection via EHRs. A hybrid approach has also been proposed, where the EHR is used to identify study endpoints (e.g., death, hospitalization, myocardial infarction, and cancer), followed by adjudication and validation of EHR findings using clinical data (e.g., electrocardiogram and laboratory data).
Validity should be defined a priori and should be specific to the endpoints of interest as well as relevant to the country or healthcare system. Validation studies should aim to assess both the consistency between EHR data and standard data collection methods, and also how identified differences influence a study’s results. Proposed uses of EHRs for registration trials and methods for their validation will likely be considered by regulatory agencies on a case-by-case basis, because of the limited experience with EHRs for this purpose at the current time. Collaboration among industry sponsors to share cumulative experiences with EHR validation studies might lead to faster acceptance by regulatory authorities.
The ESC-CRT recommends that initial efforts to integrate EHRs in clinical trials focus on a few efficacy endpoints of interest, preferably objective endpoints (e.g., all-cause or cause-specific mortality) that are less susceptible to bias or subjective interpretation. As noted above, mortality may be incompletely captured in EHRs, particularly if patients die outside of the hospital, or at another institution using a non-integrated EHR. Thus, methods to supplement endpoint ascertainment in the EHR may be necessary if data completeness is uncertain. Standardized endpoint definitions based on the EHR should be included in the study protocol and analysis plan. A narrow set of data elements for auditing should be prospectively defined to ensure the required variables which are contained in the EHR.
Early interaction between sponsors, clinical investigators, and regulators is recommended to enable robust designs for clinical trials aiming to use EHRs for endpoint ascertainment. Plans to translate Good Clinical Practice into an EHR facilitated research environment should be described. Gaps in personnel training and education should be identified and specific actions to address training deficiencies should be communicated to regulators and in place prior to the start of the trial.
Timely access to electronic health record data
The potential for delays in data access is an important consideration when EHRs are used in clinical trials. EHRs may contain data originally collected as free text that was later coded for the EHR. Thus, coded information may not be available for patient identification/recruitment during the admission. Similarly, coding may occur weeks or months after discharge. In nationally integrated systems, data availability may also be delayed. These delays may be critical depending on the purpose of data extracted from the EHR (e.g., SAE reporting, source data, or endpoints in a time-sensitive study).
Heterogeneity between systems
Patients may be treated by multiple healthcare providers who operate independently of one another. Such patients may have more than one EHR, and these EHRs may not be linked. This heterogeneity adds to the complexity of using EHRs for clinical trials, since data coordinating centres have to develop processes for interacting or extracting data from any number of different systems. Differences in quality [47], non-standardized terminology, incomplete data capture, issues related to data sharing and data privacy, lack of common data fields, and the inability of systems to be configured to communicate with each other may also be problematic. Achieving agreement on a minimum set of common data fields to enable cross communication between systems would be a major step forward towards enabling EHRs to be used in clinical trials across centers and regions [48, 49].
Data security and privacy
Privacy issues and information governance are among the most complex aspects of implementing EHRs for clinical research, in part because attitudes and regulations related to data privacy vary markedly around the world. Data security and appropriate use are high priorities, but access should not be restricted to the extent that the data are of limited usefulness. Access to EHR data by regulatory agencies will be necessary for auditing purposes in registration trials. Distributed analyses have the advantage of allowing data to remain with the individual site and under its control [39, 41].
Pre-trial planning is critical to anticipate data security issues and to develop optimal standards and infrastructure. For pivotal registration trials, patients should be informed during the consent process about how their EHRs will be used and by whom. Modified approaches to obtaining informed consent for comparative effectiveness research studies of commonly used clinical practices or interventions may be possible [50]. A general upfront consent stating that EHR data may be used for research is a proactive step that may minimize later barriers to data access, although revision of existing legislation or ethics board rules may be needed to allow this approach. Patients and the public should be recognized as important stakeholders, and they can be advocates for clinical research using EHRs and improve the quality of EHR-based research if they are educated and engaged in the process and the purpose and procedures for EHR use are transparent. Developing optimal procedures for ensuring patients that are informed and protected, balanced with minimizing barriers to research is a major consideration as EHR-based research advances.
System capabilities
EHRs for use in clinical research need a flexible architecture to accommodate studies of different interventions or disease states. EHR systems may be capable of matching eligibility criteria to relevant data fields and flagging potential trial subjects to investigators. Patient questionnaires and surveys can be linked to EHRs to provide additional context to clinical data. Pre-population of eCRFs has been proposed as a potential role for EHRs, but the proportion of fields in an EHR that can be mapped to an eCRF varies substantially across systems.
EHRs may be more suitable for pragmatic trials where data collection mirrors those variables collected in routine clinical care. Whether regulators would require collection of additional elements to support a new drug or new indication depends on the drug, intended indication, patient population, and potential safety concerns.
Sustainability
The sustainability of EHRs in clinical research will largely depend on the materialization of their promised efficiencies. Programs like the NIH Collaboratory [41] and PCORnet [39, 41], and randomized registry trials [51, 52] are demonstrating the feasibility of these more efficient approaches to clinical research. The sustainability of using EHRs for pivotal registration clinical trials will depend on regulatory acceptance of the approach and whether the efficiencies support a business case for their use.
Role of stakeholders
To make the vision of EHRs in clinical trials a reality, stakeholders should collaborate and contribute to the advancement of EHRs for research. Professional bodies, such as the ESC, can play a major role in the training and education of researchers and the public about the potential value of EHR. Clinical trialists and industry must be committed to advancing validation methodology [53]. Investigators should develop, conduct, and promote institutional EHR trials that change clinical practice; such experience may encourage EHR trial adoption by industry and the agencies. Development of core or minimal data sets could streamline the process, reduce redundancy and heterogeneity, and decrease start-up time for future EHR-based clinical trials. These and other stakeholder contributions are outlined in Table 3.
Conclusion
Electronic health records are a promising resource to improve the efficiency of clinical trials and to capitalize on novel research approaches. EHRs are useful data sources to support comparative effectiveness research and new trial designs that may answer relevant clinical questions as well as improve efficiency and reduce the cost of cardiovascular clinical research. Initial experience with EHRs has been encouraging, and accruing knowledge will continue to transform the application of EHRs for clinical research. The pace of technology has produced unprecedented analytic capabilities, but these must be pursued with appropriate measures in place to manage security, privacy, and ensure adequacy of informed consent. Ongoing programs have implemented creative solutions for these issues using distributed analyses to allow organizations to retain data control and by engaging patient stakeholders. Whether EHRs can be successfully applied to the conventional drug development in pivotal, registration trials remains to be seen and will depend on demonstration of data quality and validity, as well as realization of expected efficiencies.
References
Jackson N, Atar D, Borentain M, Breithardt G, van Eickels M, Endres M, Fraass U, Friede T, Hannachi H, Janmohamed S, Kreuzer J, Landray M, Lautsch D, Le Floch C, Mol P, Naci H, Samani N, Svensson A, Thorstensen C, Tijssen J, Vandzhura V, Zalewski A, Kirchhof P (2016) Improving clinical trials for cardiovascular diseases: a position paper from the Cardiovascular Roundtable of the European Society of Cardiology. Eur Heart J 37:747–754
Eisenstein EL, Collins R, Cracknell BS, Podesta O, Reid ED, Sandercock P, Shakhov Y, Terrin ML, Sellers MA, Califf RM, Granger CB, Diaz R (2008) Sensible approaches for reducing clinical trial costs. Clin Trials 5:75–84
Denaxas SC, Morley KI (2015) Big biomedical data and cardiovascular disease research: opportunities and challenges. European Heart Journal - Quality of Care and Clinical Outcomes 1:9–16
Hayrinen K, Saranto K, Nykanen P (2008) Definition, structure, content, use and impacts of electronic health records: a review of the research literature. Int J Med Inform 77:291–304
Appari A, Eric JM, Anthony DL (2013) Meaningful use of electronic health record systems and process quality of care: evidence from a panel data analysis of U.S. acute-care hospitals. Health Serv Res 48:354–375
Blumenthal D, Tavenner M (2010) The “meaningful use” regulation for electronic health records. N Engl J Med 363:501–504
Roumia M, Steinhubl S (2014) Improving cardiovascular outcomes using electronic health records. Curr Cardiol Rep 16:451
Doods J, Botteri F, Dugas M, Fritz F (2014) A European inventory of common electronic health record data elements for clinical trial feasibility. Trials 15:18
Collins FS, Hudson KL, Briggs JP, Lauer MS (2014) PCORnet: turning a dream into reality. J Am Med Inform Assoc 21:576–577
James S, Rao SV, Granger CB (2015) Registry-based randomized clinical trials–a new clinical trial paradigm. Nat Rev Cardiol 12:312–316
Krumholz HM, Normand SL, Wang Y (2014) Trends in hospitalizations and outcomes for acute cardiovascular disease and stroke, 1999-2011. Circulation 130:966–975
Hlatky MA, Ray RM, Burwen DR, Margolis KL, Johnson KC, Kucharska-Newton A, Manson JE, Robinson JG, Safford MM, Allison M, Assimes TL, Bavry AA, Berger J, Cooper-DeHoff RM, Heckbert SR, Li W, Liu S, Martin LW, Perez MV, Tindle HA, Winkelmayer WC, Stefanick ML (2014) Use of Medicare data to identify coronary heart disease outcomes in the Women’s Health Initiative. Circ Cardiovasc Qual Outcomes 7:157–162
Chung SC, Gedeborg R, Nicholas O, James S, Jeppsson A, Wolfe C, Heuschmann P, Wallentin L, Deanfield J, Timmis A, Jernberg T, Hemingway H (2014) Acute myocardial infarction: a comparison of short-term survival in national outcome registries in Sweden and the UK. Lancet 383:1305–1312
Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF (2001) The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR): building a national clinical data repository. J Am Coll Cardiol 37:2240–2245
Scholte op Reimer W, Gitt A, Boersma E, Simoons Me (2006) Cardiovascular diseases in Europe. Euro Heart Survey−2006. European Society of Cardiology, . Sophia Antipolis
Ferrari R (2010) EURObservational research programme. Eur Heart J 31:1023–1031
Smaha LA (2004) The American Heart Association Get With The Guidelines program. Am Heart J 148:S46–S48
Krumholz HM (2014) Big data and new knowledge in medicine: the thinking, training, and tools needed for a learning health system. Health Aff (Millwood) 33:1163–1170
Wood R, Clark D, King A, Mackay D, Pell J (2013) Novel cross-sectoral linkage of routine health and education data at an all-Scotland level: a feasibility study. Lancet 382(Supplement 3):S10
Cederholm S, Hill G, Asiimwe A, Bate A, Bhayat F, Persson BG, Bergvall T, Ansell D, Star K, Noren GN (2015) Structured assessment for prospective identification of safety signals in electronic medical records: evaluation in the health improvement network. Drug Saf 38:87–100
Trifiro G, Fourrier-Reglat A, Sturkenboom MC, Diaz AC, Van Der Lei J (2009) The EU-ADR project: preliminary results and perspective. Stud Health Technol Inform 148:43–49
Eichler HG, Pignatti F, Flamion B, Leufkens H, Breckenridge A (2008) Balancing early market access to new drugs with the need for benefit/risk data: a mounting dilemma. Nat Rev Drug Discov 7:818–826
Goedecke T, Arlett P (2014) A Description of the European Network of Centres for pharmacoepidemiology and pharmacovigilance as a global resource for pharmacovigilance and pharmacoepidemiology. Mann’s pharmacovigilance. Wiley, New York, pp 403–408
Ball R, Robb M, Anderson SA, Dal Pan G (2016) The FDA’s sentinel initiative-A comprehensive approach to medical product surveillance. Clin Pharmacol Ther 99:265–268
Staffa JA, Dal Pan GJ (2012) Regulatory innovation in postmarketing risk assessment and management. Clin Pharmacol Ther 91:555–557
Peterson ED, Shah BR, Parsons L, Pollack CV Jr, French WJ, Canto JG, Gibson CM, Rogers WJ (2008) Trends in quality of care for patients with acute myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J 156:1045–1055
Chan PS, Maddox TM, Tang F, Spinler S, Spertus JA (2011) Practice-level variation in warfarin use among outpatients with atrial fibrillation (from the NCDR PINNACLE program). Am J Cardiol 108:1136–1140
Maddox TM, Chan PS, Spertus JA, Tang F, Jones P, Ho PM, Bradley SM, Tsai TT, Bhatt DL, Peterson PN (2014) Variations in coronary artery disease secondary prevention prescriptions among outpatient cardiology practices: insights from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol 63:539–546
Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallentin L (2010) The Swedish Web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart 96:1617–1621
Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Aguilar JC, Dietz R, Gavazzi A, Hobbs R, Korewicki J, Madeira HC, Moiseyev VS, Preda I, van Gilst WH, Widimsky J, Freemantle N, Eastaugh J, Mason J (2003) The EuroHeart Failure survey programme: a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J 24:442–463
Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, Hochadel M, Komajda M, Lassus J, Lopez-Sendon JL, Ponikowski P, Tavazzi L (2006) EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J 27:2725–2736
Tofield A (2010) EURObservational research programme. Eur Heart J 31:1023–1031
McNamara RL, Herrin J, Bradley EH, Portnay EL, Curtis JP, Wang Y, Magid DJ, Blaney M, Krumholz HM (2006) Hospital improvement in time to reperfusion in patients with acute myocardial infarction, 1999 to 2002. J Am Coll Cardiol 47:45–51
Kopcke F, Trinczek B, Majeed RW, Schreiweis B, Wenk J, Leusch T, Ganslandt T, Ohmann C, Bergh B, Rohrig R, Dugas M, Prokosch HU (2013) Evaluation of data completeness in the electronic health record for the purpose of patient recruitment into clinical trials: a retrospective analysis of element presence. BMC Med Inform Decis Mak 13:37
Thadani SR, Weng C, Bigger JT, Ennever JF, Wajngurt D (2009) Electronic screening improves efficiency in clinical trial recruitment. J Am Med Inform Assoc 16:869–873
De Moor G, Sundgren M, Kalra D, Schmidt A, Dugas M, Claerhout B, Karakoyun T, Ohmann C, Lastic PY, Ammour N, Kush R, Dupont D, Cuggia M, Daniel C, Thienpont G, Coorevits P (2015) Using electronic health records for clinical research: the case of the EHR4CR project. J Biomed Inform 53:162–173
Fordyce CB, Roe MT, Ahmad T, Libby P, Borer JS, Hiatt WR, Bristow MR, Packer M, Wasserman SM, Braunstein N, Pitt B, DeMets DL, Cooper-Arnold K, Armstrong PW, Berkowitz SD, Scott R, Prats J, Galis ZS, Stockbridge N, Peterson ED, Califf RM (2015) Cardiovascular drug development: is it dead or just hibernating? J Am Coll Cardiol 65:1567–1582
New JP, Bakerly ND, Leather D, Woodcock A (2014) Obtaining real-world evidence: the Salford Lung Study. Thorax 69:1152–1154
Fleurence RL, Curtis LH, Califf RM, Platt R, Selby JV, Brown JS (2014) Launching PCORnet, a national patient-centered clinical research network. J Am Med Inform Assoc 21:578–582
Hernandez AF, Fleurence RL, Rothman RL (2015) The ADAPTABLE Trial and PCORnet: shining light on a new research paradigm. Ann Intern Med 163:635–636
Curtis LH, Brown J, Platt R (2014) Four health data networks illustrate the potential for a shared national multipurpose big-data network. Health Aff (Millwood) 33:1178–1186
Jha AK, DesRoches CM, Campbell EG, Donelan K, Rao SR, Ferris TG, Shields A, Rosenbaum S, Blumenthal D (2009) Use of electronic health records in U.S. hospitals. N Engl J Med 360:1628–1638
Hersh WR, Weiner MG, Embi PJ, Logan JR, Payne PR, Bernstam EV, Lehmann HP, Hripcsak G, Hartzog TH, Cimino JJ, Saltz JH (2013) Caveats for the use of operational electronic health record data in comparative effectiveness research. Med Care 51:S30–S37
Brennan L, Watson M, Klaber R, Charles T (2012) The importance of knowing context of hospital episode statistics when reconfiguring the NHS. BMJ 344:e2432
Green SM (2013) Congruence of disposition after emergency department intubation in the National Hospital Ambulatory Medical Care Survey. Ann Emerg Med 61:423–426
Weiskopf NG, Weng C (2013) Methods and dimensions of electronic health record data quality assessment: enabling reuse for clinical research. J Am Med Inform Assoc 20:144–151
Elnahal SM, Joynt KE, Bristol SJ, Jha AK (2011) Electronic health record functions differ between best and worst hospitals. Am J Manag Care 17:e121–e147
Flynn MR, Barrett C, Cosio FG, Gitt AK, Wallentin L, Kearney P, Lonergan M, Shelley E, Simoons ML (2005) The Cardiology Audit and Registration Data Standards (CARDS), European data standards for clinical cardiology practice. Eur Heart J 26:308–313
Simoons ML, van der Putten N, Wood D, Boersma E, Bassand JP (2002) The Cardiology Information System: the need for data standards for integration of systems for patient care, registries and guidelines for clinical practice. Eur Heart J 23:1148–1152
Sugarman J, Califf RM (2014) Ethics and regulatory complexities for pragmatic clinical trials. JAMA 311:2381–2382
Frobert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, Aasa M, Angeras O, Calais F, Danielewicz M, Erlinge D, Hellsten L, Jensen U, Johansson AC, Karegren A, Nilsson J, Robertson L, Sandhall L, Sjogren I, Ostlund O, Harnek J, James SK (2013) Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med 369:1587–1597
Hess CN, Rao SV, Kong DF, Aberle LH, Anstrom KJ, Gibson CM, Gilchrist IC, Jacobs AK, Jolly SS, Mehran R, Messenger JC, Newby LK, Waksman R, Krucoff MW (2013) Embedding a randomized clinical trial into an ongoing registry infrastructure: unique opportunities for efficiency in design of the Study of Access site For Enhancement of Percutaneous Coronary Intervention for Women (SAFE-PCI for Women). Am Heart J 166:421–428
Barry SJ, Dinnett E, Kean S, Gaw A, Ford I (2013) Are routinely collected NHS administrative records suitable for endpoint identification in clinical trials? Evidence from the West of Scotland Coronary Prevention Study. PLoS One 8:e75379
Acknowledgments
This paper was generated from discussions during a cardiovascular round table (CRT) Workshop organized on 23–24 April 2015 by the European Society of Cardiology (ESC). The CRT is a strategic forum for high-level dialogues between academia, regulators, industry, and ESC leadership to identify and discuss key strategic issues for the future of cardiovascular health in Europe and other parts of the world. We acknowledge Colin Freer for his participation in the meeting. This article reflects the views of the authors and should not be construed to represent FDA’s views or policies. The opinions expressed in this paper are those of the authors and cannot be interpreted as the opinion of any of the organizations that employ the authors. MRC’s salary is supported by the National Institute for Health Research (NIHR) Cardiovascular Biomedical Research Unit at the Royal Brompton Hospital, London, UK.
Conflict of interest
Martin R. Cowie: Research grants from ResMed, Boston Scientific, and Bayer; personal fees from ResMed, Boston Scientific, Bayer, Servier, Novartis, St. Jude Medical, and Pfizer. Juuso Blomster: Astra Zeneca employee. Lesley Curtis: Funding from FDA for work with the Mini-Sentinel program and from PCORI for work with the PCORnet program. Sylvie Duclaux: None. Ian Ford: None. Fleur Fritz: None. Samantha Goldman: None. Salim Janmohamed: GSK employee and shareholder. Jörg Kreuzer: Employee of Boehringer-Ingelheim. Mark Leenay: Employee of Optum. Alexander Michel: Bayer employee and shareholder. Seleen Ong: Employee of Pfizer. Jill Pell: None. Mary Ross Southworth: None. Wendy Gattis Stough: Consultant to European Society of Cardiology, Heart Failure Association of the European Society of Cardiology, European Drug Development Hub, Relypsa, CHU Nancy, Heart Failure Society of America, Overcome, Stealth BioTherapeutics, Covis Pharmaceuticals, University of Gottingen, and University of North Carolina. Martin Thoenes: Employee of Edwards Lifesciences. Faiez Zannad: Personal fees from Boston Scientific, Servier, Pfizer, Novartis, Takeda, Janssen, Resmed, Eli Lilly, CVRx, AstraZeneca, Merck, Stealth Peptides, Relypsa, ZS Pharma, Air Liquide, Quantum Genomics, Bayer for Steering Committee, Advisory Board, or DSMB member. Andrew Zalewski: Employee of GSK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Cowie, M.R., Blomster, J.I., Curtis, L.H. et al. Electronic health records to facilitate clinical research. Clin Res Cardiol 106, 1–9 (2017). https://doi.org/10.1007/s00392-016-1025-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-016-1025-6