Abstract.
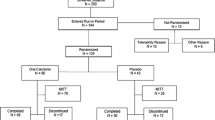
The aim of this study was to assess the efficacy and safety of nasal spray and subcutaneous formulations of salmon calcitonin. Two-hundred-four patients, 27 males and 177 females, aged 72 years on average, with a recent, painful, vertebral crush fracture were given either 50 IU/day of subcutaneous salmon calcitonin (SCSCT, 102 patients) or 200 IU/day of intranasal salmon calcitonin (INSCT, 102 patients) for 30 consecutive days, according to a double-blind, double-placebo design. The two-sided 95% confidence interval of the difference between the two formulations for the pain on D30 assessed by Huskisson's Visual Analogue Scale (VAS) [−5.3 mm, 7.9 mm] was included in the [−10 mm, 10 mm] reference interval. Equivalence of the two formulations, was demonstrated. At the end of the study, the 95% confidence intervals of VAS of both treatment groups were included in the [0 mm, 30 mm] interval, which is considered to be clinically pertinent. Relief was obtained in less than 10 days for more than 50% of patients. The urinary hydroxyproline/creatinine and calcium/creatinine ratios remained constant between D1 and D30 with both formulations. General safety was comparable between the two formulations. Local safety of INSCT was similar to that of its placebo.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 13 August 1996 / Accepted: 31 December 1996
Rights and permissions
About this article
Cite this article
Combe, B., Cohen, C. & Aubin, F. Equivalence of Nasal Spray and Subcutaneous Formulations of Salmon Calcitonin. Calcif Tissue Int 61, 10–15 (1997). https://doi.org/10.1007/s002239900284
Published:
Issue Date:
DOI: https://doi.org/10.1007/s002239900284