Abstract
Summary
The comparative effectiveness of alendronate and risedronate has received limited evaluation. Among 19,063 new users of bisphosphonates, risedronate users had a higher relative rate of hip fracture compared to alendronate users, but the difference in absolute fracture rate was small. We conclude that the agents have comparable efficacy.
Introduction
Bisphosphonates differ in their in vitro potency, avidity for bone, and rapidity of onset in clinical trials. To address potential differences between bisphosphonates in comparative effectiveness, we compared new users of alendronate and risedronate to determine if there were differences in the risk of clinical fractures at 1 year and beyond.
Methods
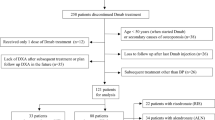
Using claims data from a U.S. health care organization, we identified new, adherent users of weekly alendronate or risedronate and assessed subsequent fractures. We calculated fracture incidence rate differences and ratios between the two agents.
Results
There were no significant differences in fracture rates between alendronate users (n = 12,956) and risedronate users (n = 6,107) at 1 year. Using all available data, the rate of hip fracture was higher among risedronate users compared to alendronate users (absolute rate difference approximately five per 1,000 person-years). Risedronate users had a higher relative rate (RR) of hip fracture (RR = 1.77, 95% CI 1.15–2.74) and similar rates of clinical vertebral and nonvertebral fractures compared to alendronate users.
Conclusions
The absolute rate of clinical fractures among alendronate and risedronate users was similar both at 1 year and beyond, suggesting comparable effectiveness between agents.

Similar content being viewed by others
References
Black DM, Cummings SR, Karpf DB et al (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 348:1535–1541
Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures. J Am Med Assoc 280:2077–2082
McClung MR, Geusens P, Miller PD et al (2001) Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 344(5):333–340
Harris ST, Watts NB, Genant HK et al (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA 282:1344–1352
Reginster J, Minne HW, Sorensen OH et al (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 11(1):83–91
Bonnick S, Saag KG, Kiel DP et al (2006) Comparison of weekly treatment of postmenopausal osteoporosis with alendronate versus risedronate over two years. J Clin Endocrinol Metab 91(7):2631–2637
Dunford JE, Thompson K, Coxon FP et al (2001) Structure–activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther 296(2):235–242
Nancollas GH, Tang R, Phipps RJ et al (2006) Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 38(5):617–627
Green JR, Muller K, Jaeggi KA (1994) Preclinical pharmacology of CGP 42′446, a new, potent, heterocyclic bisphosphonate compound. J Bone Miner Res 9(5):745–751
Silverman SL, Watts NB, Delmas PD, Lange JL, Lindsay R (2007) Effectiveness of bisphosphonates on nonvertebral and hip fractures in the first year of therapy: the risedronate and alendronate (REAL) cohort study. Osteoporos Int 18(1):25–34
Saag K, Lindsay R, Kriegman A, Beamer E, Zhou W (2007) A single zoledronic acid infusion reduces bone resorption markers more rapidly than weekly oral alendronate in postmenopausal women with low bone mineral density. Bone 40(5):1238–1243
McClung M, Recker R, Miller P et al (2007) Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone 41(1):122–128
Curtis JR, Mudano AS, Solomon DH, Xi J, Melton ME, Saag KG (2008) Identification and validation of vertebral compression fractures using administrative claims data. Med Care (in press)
Curtis JR, Westfall AO, Allison J, Freeman A, Kovac SH, Saag KG (2006) Agreement and validity of pharmacy data versus self-report for use of osteoporosis medications among chronic glucocorticoid users. Pharmacoepidemiol Drug Saf 15(10):710–718
Cramer JA, Amonkar MM, Hebborn A, Altman R (2005) Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin 21(9):1453–1460
Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45(6):613–619
Disclosures
JC: research grants: Novartis, Amgen, Merck, Proctor & Gamble, Eli Lilly; consulting: Roche; speakers bureau: Merck, Procter and Gamble, Eli Lilly, Roche. AW: research grants: Novartis. HC: research grants: Amgen. KGS: research grants: Novartis, Amgen, Aventis, Merck, Procter & Gamble, Eli Lilly, Roche; consulting or speaking: Merck, Proctor and Gamble, Eli Lilly, Roche, Novartis, Amgen. ED: research grants: Amgen.
Author information
Authors and Affiliations
Corresponding author
Additional information
Some of the investigators (JRC and KGS) receive salary support from the National Institutes of Health (AR053351, AR052361) and the Arthritis Foundation (JRC).
Rights and permissions
About this article
Cite this article
Curtis, J.R., Westfall, A.O., Cheng, H. et al. RisedronatE and ALendronate Intervention over Three Years (REALITY): minimal differences in fracture risk reduction. Osteoporos Int 20, 973–978 (2009). https://doi.org/10.1007/s00198-008-0772-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-008-0772-2