Abstract
Aims/hypothesis
This study was designed to test the hypothesis that low plasma vitamin B12 concentrations combined with high folate concentrations in pregnancy are associated with a higher incidence of gestational diabetes (GDM) and later diabetes.
Methods
Women (N = 785) attending the antenatal clinics of one hospital in Mysore, India, had their anthropometry, insulin resistance (homeostasis model assessment-2) and glucose tolerance assessed at 30 weeks’ gestation (100 g oral glucose tolerance test; Carpenter–Coustan criteria) and at 5 years after delivery (75 g OGTT; WHO, 1999). Gestational vitamin B12 and folate concentrations were measured in stored plasma samples.
Results
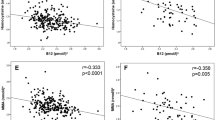
Low vitamin B12 concentrations (<150 pmol/l, B12 deficiency) were observed in 43% of women and low folate concentrations (<7 nmol/l) in 4%. B12-deficient women had higher body mass index (p < 0.001), sum of skinfold thickness (p < 0.001), insulin resistance (p = 0.02) and a higher incidence of GDM (8.7% vs 4.6%; OR 2.1, p = 0.02; p = 0.1 after adjusting for BMI) than non-deficient women. Among B12-deficient women, the incidence of GDM increased with folate concentration (5.4%, 10.5%, 10.9% from lowest to highest tertile, p = 0.04; p for interaction = 0.2). Vitamin B12 deficiency during pregnancy was positively associated with skinfold thickness, insulin resistance (p < 0.05) and diabetes prevalence at 5 year follow-up (p = 0.009; p = 0.008 after adjusting for BMI). The association with diabetes became non-significant after excluding women with previous GDM (p = 0.06).
Conclusions/interpretation
Maternal vitamin B12 deficiency is associated with increased adiposity and, in turn, with insulin resistance and GDM. Vitamin B12 deficiency may be an important factor underlying the high risk of ‘diabesity’ in south Asian Indians.
Similar content being viewed by others
Introduction
The prevalence of type 2 diabetes is increasing rapidly in countries such as India. Type 2 diabetes tends to appear at a younger age in Indians than in white populations in high-income countries [1]. One consequence of this is a high incidence of gestational diabetes (GDM), reaching 10–20% in some urban studies [2]. A major contributory factor to this is the characteristic phenotype of Indians, which includes lower muscle mass, a higher percentage of body fat and higher insulin resistance compared with other populations [3].
Although the cause of this adverse phenotype is unknown, a role for intrauterine undernutrition has been suggested [3]. Specifically, in the Pune Maternal Nutrition Study (PMNS), children born to mothers with low plasma vitamin B12 concentrations were more adipose and insulin resistant than children born to mothers with normal vitamin B12, especially if the mother had normal or high folate concentrations [4]. Folate and vitamin B12 are essential for several important methylation processes involving synthesis, repair and/or regulation of nucleic acids, proteins, membrane phospholipids, neurotransmitters and epigenetic modifications. Deficiency of these vitamins is associated with wide-ranging multi-system abnormalities, including megaloblastic anaemia, neurological disorders and birth defects [5]. The PMNS authors hypothesised a general phenomenon whereby the susceptibility of Indians to adiposity and diabetes (‘diabesity’) might result from widespread vitamin B12 deficiency, which may be further exacerbated by recent improvement in folate nutrition [4]. Indeed, a high prevalence of vitamin B12 deficiency has been demonstrated in various subgroups of the Indian population [4, 6–9]. We tested this hypothesis in pregnant women who participated in the Parthenon Study, which investigated the effect of GDM on newborn size and future risk of diabetes [10, 11]. We hypothesised that maternal vitamin B12 deficiency is associated with increased adiposity, insulin resistance and impaired glucose tolerance in the mother herself, and that these outcomes are exaggerated by adequate or high folate status.
Methods
A scheme of the number of participants and the investigations done at different time points is illustrated in Fig. 1.
Pregnancy
During 1997–1998, 830 women booking consecutively into the antenatal clinic of the Holdsworth Memorial Hospital (HMH) in Mysore, south India, and matching our eligibility criteria (no known history of diabetes, intention to deliver at HMH, singleton pregnancy) had a 100 g 3 h OGTT at 30 ± 2 weeks’ gestation after an overnight fast [10]. Weight, height and skinfold thickness (biceps, triceps, subscapular and suprailiac) were measured using standardised methods [12]. Socioeconomic status (SES) was assessed using the Kuppuswamy score, a questionnaire which derives a score based on the education, occupation and the income of the head of the family [13]; a higher score indicates higher SES. The majority of our women belonged to middle or lower-middle social classes (score: 5–25).
Plasma glucose was measured using a standard hexokinase method, and insulin using a one-step chemiluminescent immunoenzymatic assay (Sanofi Pasteur Diagnostics, Marnes la Coquette, France). Complete OGTT results were available for 785 women. GDM was diagnosed in 49 women (6.2%) using the Carpenter–Coustan criteria [14], the standard method in clinical use in the HMH. Insulin resistance was estimated using the updated homeostasis model assessment equation (HOMA-2) from an online HOMA calculator [15]. The women’s own consultant obstetricians managed their further clinical care.
A total of 630 women delivered live, normal babies in HMH. There were 41 women diagnosed with GDM, ten of whom received insulin treatment during pregnancy, but none of whom was on insulin or oral hypoglycaemic agents at the time of discharge from the hospital.
The hospital ethical committee approved the study, and informed verbal consent was obtained from all women.
Vitamin B12 and folic acid supplements
It was routine for general practitioners and obstetricians to prescribe folic acid and/or multivitamin supplements to pregnant women. Supplements taken by the women were recorded at recruitment but not subsequently at 30 weeks’ gestation, when blood samples were taken, or at term.
Vitamin B12 and folate during pregnancy
We used stored fasting plasma samples to measure vitamin B12 and folate in 774 of the 785 women who had complete OGTT data in pregnancy. The samples had been stored in the freezer (−80°C for 8 years) within 1 h of sampling, and were transferred on dry ice for laboratory analysis at the Diabetes Research Centre, KEM Hospital, Pune, India, using microbiological assays [16, 17]. Intra- and interassay coefficients of variations were <8% for both assays. As there is no specified cut-off for pregnancy, we defined vitamin B12 deficiency as a concentration <150 pmol/l and folate deficiency as a concentration <7 nmol/l based on the values generally used in a normal population [9, 18].
Follow-up
Follow-up of the women was based on the follow-up of their offspring [11]. Twenty-five children died between birth and 5 years, seven children were excluded after birth because of medical reasons, and 43 families either refused follow-up or moved away from Mysore. Five years later, we were able to follow 555 women, 29 of whom were excluded from the current study because of recent pregnancy (within the previous 6 months). The remaining 526 women had a 2 h, 75 g OGTT; 519 of these women, for whom vitamin B12 and folate concentrations were measured (35 met criteria for GDM), completed the study. Detailed anthropometry was performed and systolic (SBP) and diastolic blood pressures (DBP) were measured using an automated BP monitor (CRITIKON, DINAMAP model 8100, Tampa, FL, USA). Plasma glucose (glucose oxidase–peroxidase method), triacylglycerols (glycerol 3 phosphate oxidase-peroxidase method) and HDL-cholesterol (direct HDL-cholesterol method) were measured on an autoanalyser (Alcyon 300; Abbott Laboratories, Abbott Park, IL, USA), and insulin was measured using a time-resolved, fluoroimmunoassay (DELFIA) method (PerkinElmer Life and Analytical Sciences, Wallac Qy, Turku, Finland) at the Diabetic Research Centre, Pune, India.
Diabetes was defined as a fasting glucose concentration ≥7.0 and/or 120 min glucose ≥11.1 mmol/l [19]. Women were also classified as having diabetes if they had been diagnosed by a doctor as having diabetes since the index pregnancy. Impaired glucose tolerance (IGT) was a fasting glucose concentration <7.0 mmol/l and 120 min glucose ≥7.8 mmol/l but <11.1 mmol/l. Impaired fasting glucose (IFG) was defined as fasting glucose ≥6.1 mmol/l but <7.0 mmol/l [19].
Metabolic syndrome was defined by the International Diabetes Federation (IDF) criteria recommended for south Asian women [20]. Waist circumference ≥80 cm, and any two of the following: triacylglycerol ≥1.7 mmol/l; HDL-cholesterol <1.29 mmol/l; SBP ≥130 or DBP ≥85 or having treatment for hypertension; fasting glucose ≥5.6 mmol/l; or type 2 diabetes.
Statistical methods
The distributions of HOMA-2 and vitamin B12 concentrations were skewed; these data were log-transformed for analysis where required. The main exposures of interest were the vitamin B12 deficiency (yes/no), and plasma vitamin B12 and folate concentrations. Other confounding exposures such as maternal age, parity, religion, family history of diabetes and SES were used as covariates in the multiple regression models. The outcomes of interest were anthropometry, insulin resistance and the incidence of GDM during pregnancy, and anthropometry, insulin resistance and the prevalence of diabetes and metabolic syndrome at follow-up. Associations of maternal vitamin B12 and folate concentrations with anthropometry and HOMA-2 during pregnancy and at follow-up were examined using linear regression analysis, and with the incidence of GDM, and the prevalence of diabetes and metabolic syndrome at follow-up, using logistic regression analysis. Interaction terms were used to test for modification by folate status of associations between vitamin B12 status and the several diabetes-related outcomes by using vitamin B12 as two groups (deficiency and normal groups), and tertiles of folate concentrations. p values <0.05 were considered significant. All statistical analyses were performed using SPSS V16.
Results
Three-hundred and thirty-five women (43%) had vitamin B12 deficiency, while only 34 (4%) had folate deficiency (Table 1). Vitamin B12 deficiency was higher among Hindu women (50.7%, median B12 = 148.5 pmol/l) than Muslims (31.8%, 180.0 pmol/l) and others (35.6%, 186.0 pmol/l); folate concentrations were lowest among Muslim women (24.2 nmol/l; Hindus 37.5 mmol/l; others 46.9 nmol/l). Vitamin B12 concentrations were unrelated to SES, parity or family history of diabetes. Higher SES (p < 0.001), presence of a family history of diabetes (p = 0.003), and lower parity (p < 0.001) were associated with higher folate concentrations.
At recruitment, 239 (31%) women reported taking multivitamin supplements containing both vitamin B12 and folic acid, and 60 (8%) reported taking folic acid alone. Supplement use was not significantly associated with vitamin B12 and folate concentrations.
Vitamin B12, folate and outcomes during pregnancy
Vitamin B12 concentrations were inversely associated with the women’s BMI and sum of skinfold thickness (Table 2). Vitamin B12 deficiency was associated with higher BMI, larger sum of skinfold thickness, higher HOMA-2 and an increased risk of GDM after adjusting for age, religion, SES, parity and family history of diabetes. Gestational diabetes was two times more frequent in vitamin B12-deficient women compared with non-deficient women. The associations with GDM became non-significant after adjusting for BMI (OR 1.1, p = 0.1) or sum of skinfold thickness (p = 0.09).
Vitamin B12-deficient women were also more likely to be in the highest quartile for measures of adiposity (BMI: OR 1.9, 95% CI 1.4–2.6, p < 0.001, sum of skinfold thickness: OR 1.6, 95% CI 1.2–2.3, p = 0.004). Folate concentrations were not related to the women’s adiposity, insulin resistance or the incidence of GDM (Table 2).
In women with vitamin B12 deficiency, the incidence of GDM increased across tertiles of the folate distribution (5.4% (n = 7), 10.5% (n = 12), 10.9% (n = 10)) from lowest to highest tertile (p = 0.1; p = 0.04 after adjusting for age, religion, SES, parity and family history of diabetes). There was no significant interaction between vitamin B12 deficiency and folate concentrations for the incidence of GDM (p = 0.2). Insulin resistance decreased in non-deficient women (β = −0.09, p = 0.02) from the lowest to highest tertiles of folate concentrations, there was no significant association between folate and insulin resistance in B12-deficient women (β = 0.04, p = 0.4, p for interaction = 0.06). There was no interaction between vitamin B12 deficiency and folate concentrations for any other outcomes of interest.
Vitamin B12, folate and outcomes at follow-up
Women who were lost to follow-up after 5 years (n = 255) were significantly younger, lighter, thinner and less adipose during pregnancy (Table 1). They also had lower HOMA-2, folate concentrations and lower SES. There were no significant differences in vitamin B12 concentrations and the prevalence of vitamin B12 deficiency.
Twenty-one women acquired diabetes after pregnancy, including 12 with previous GDM. Two of them were diagnosed between pregnancy and follow-up, and 19 more were diagnosed from OGTT at follow-up.
Of the components of metabolic syndrome, 297 (57%) met the IDF criteria for high waist circumference, 26 (5%) for high BP, 207 (40%) for increased glucose variables, 87 (17%) for elevated triacylglycerols and 393 (76%) women for low HDL-cholesterol. One-hundred and forty-six women (28%) were classified as having metabolic syndrome.
Gestational vitamin B12 deficiency was significantly positively associated with maternal adiposity 5 years later, HOMA-2 and the prevalence of diabetes and metabolic syndrome (Table 3). The association with diabetes remained significant even after adjusting for gestational BMI (p = 0.009) or sum of skinfold thickness (p = 0.006). No significant associations were seen with the prevalence of IGT or IFG. There were no associations between plasma folate concentrations and 5 year outcomes, and no significant interactions between B12 deficiency and folate concentrations.
After excluding the 35 women who had GDM, gestational vitamin B12 deficiency was significantly associated with higher BMI (p < 0.001) and sum of skinfold thickness (p = 0.001). There was a non-significant association between vitamin B12 deficiency and higher insulin resistance (0.87 vs 0.78, p = 0.08), and a higher prevalence of diabetes (3.0% vs <1%; OR 5.2, 95% CI 0.95–28.2, p = 0.06) and metabolic syndrome (28.9% vs 23.7%; OR 1.4, 95% CI 0.9–2.2, p = 0.1). Among women who had GDM, ten (50%) of those with vitamin B12 deficiency during pregnancy were diagnosed with diabetes at follow-up, compared with three (20%) with normal vitamin B12 concentrations.
Discussion
We observed a high prevalence of vitamin B12 deficiency in pregnant women attending one maternity hospital in south India. Folate deficiency was rare; plasma folate concentrations as a group were normal to high. Vitamin B12 deficiency was associated with adiposity, insulin resistance and diabetes both during pregnancy and 5 years later. The associations with gestational diabetes appeared to be explained by increased adiposity, as indicated by BMI and sum of skinfold thickness. Although the risk of GDM was highest among women with B12 deficiency and adequate folate concentrations, the test for interaction did not support our hypothesis that high/adequate folate status exacerbates the effects of B12 deficiency on the diabetes risk.
Earlier studies have observed a high prevalence of vitamin B12 deficiency among Indians [6–9]. Consistent with these results, more than 40% of our women were vitamin B12 deficient while only 4% had low plasma folate concentrations. Hindu women, who are likely to be mostly lacto-vegetarian with infrequent meat consumption in our population, had the lowest vitamin B12 concentrations; Muslim women, who are mainly non-vegetarian with minimal consumption of vegetables, had the lowest folate concentrations. As our study was not originally designed to examine vitamin B12 and folate status, we recorded supplement use only at recruitment. Women not on supplements at recruitment may have been prescribed them later in pregnancy, while those prescribed supplements in early pregnancy may have stopped taking them by 30 weeks. This may be the reason for no significant association between supplement intake and vitamin concentrations.
The associations of vitamin B12 deficiency with insulin resistance and GDM were reduced after adjusting for BMI, suggesting a mediating role for adiposity. This is the first description of an association between vitamin B12 deficiency, adiposity and its associated disorders (‘diabesity’). One interpretation could be that vitamin B12 deficiency promotes adiposity. Another possibility is that obesity/adiposity lowers plasma vitamin B12 concentrations. Several studies have reported decreased bio-availability of micronutrients, especially fat-soluble vitamins and folate, in obese/overweight individuals [21–23]. The mechanisms suggested are decreased absorption or dietary intake, increased catabolism, and sequestration in adipose tissue. A poor-quality dietary intake, rich in energy content but low in micronutrients, may result in both adiposity and low B12 levels. If this was the explanation for the association we observed between maternal adiposity and vitamin B12 deficiency, we would also expect to see an association between adiposity and folate concentration. Moreover, vitamin D, a fat-soluble vitamin that is commonly decreased in obese individuals, was not associated with adiposity in our women [24].
The association between B12 deficiency and diabetes at follow-up was independent of adiposity, but not of GDM. As the association between B12 deficiency and GDM was related to adiposity, the above association may be due to residual confounding. As the prevalence of diabetes was very low in our young non-GDM women, a statistical association with B12 deficiency was difficult to show. However, even in women without GDM, the incidence of diabetes tended to increase with vitamin B12 deficiency. We speculate that there may be additional mechanisms, apart from adiposity, for the association between low vitamin B12 and diabetes.
Insulin resistance and the prevalence of GDM were highest among women with a combination of vitamin B12 deficiency and adequate/high folate concentrations. As discussed in the PMNS paper there are plausible biochemical reasons why vitamin B12 deficiency could cause increased adiposity, especially in the presence of high folate [4]. In vitamin B12 deficiency cellular folate is trapped as inactive 5-methyltetrahydrofolate [25]. This results in impaired methionine, and thus protein synthesis, which may hinder lean tissue deposition. Decreased conversion of methylmalonic acid to succinyl coA, for which vitamin B12 acts as a rate-limiting coenzyme, results in the accumulation of methylmalonic acid, and may increase lipogenesis and insulin resistance [4]. Although folate does not participate in these pathways, it has recently been shown that in vitamin B12 deficiency higher folate is associated with high concentrations of homocysteine and methylmalonic acid [26].
In our study, insulin resistance was positively related to folate concentration in B12-deficient women (non-significant association), and negatively related to folate in non-deficient women. This is analogous to data from a recent study among elderly people in the USA showing that in the presence of vitamin B12 deficiency, a high folate concentration was associated with anaemia and cognitive impairment, but with protection against cognitive impairment when vitamin B12 status was normal [27]. The possible implications are that folate may have adverse effects in vitamin B12 deficiency, or vitamin B12 status may need to be normal for optimum folate actions. Our study and the Pune study suggest that there may be a similar phenomenon with insulin resistance. However, there was no statistically significant interaction between vitamin B12 and folate in either study.
The major strength of this large prospective cohort study was the availability of both nutritional and metabolic measurements during pregnancy, and follow-up measurements after 5 years. Although the micronutrient assays were performed using stored samples, vitamin B12 and folate have been shown to be stable in long-term storage at lower temperatures [28]. The study was based in one single hospital; however, the women belonged to different religions, and had different socio-cultural backgrounds and dietary habits that were representative of the Mysore population. The dietary preferences of the majority of Hindu women were as described in other studies, thus rendering our data relevant to other parts of India [4]. Though vitamin B12 concentrations are difficult to interpret in pregnancy, because of haemodilution and raised glomerular filtration rate, and there is no agreed cut-off for deficiency; a similar definition has been used in other studies [29]. Concentrations of homocysteine and methylmalonic acid, more specific and sensitive indicators of vitamin B12 deficiency, were not measured in our study and thus we may have underestimated the prevalence of deficiency. Vitamin status at follow-up, which might have important effects on adiposity, insulin resistance and the prevalence of diabetes, was not measured. Another limitation was that information on exposures such as dietary intake and physical activity level, which may have significant impact on plasma vitamin levels, and adiposity and insulin resistance was not collected.
Conclusion
Our findings suggest a link between vitamin B12 deficiency and adiposity and diabetes (‘diabesity’) in pregnant women. Further studies are needed to confirm our findings.
Abbreviations
- DBP:
-
Diastolic blood pressure
- GDM:
-
Gestational diabetes mellitus
- HMH:
-
Holdsworth Memorial Hospital
- HOMA:
-
Homeostasis model assessment
- IDF:
-
International Diabetes Federation
- IFG:
-
Impaired fasting glucose
- IGT:
-
Impaired glucose tolerance
- PMNS:
-
Pune Maternal Nutrition Study
- SBP:
-
Systolic blood pressure
- SES:
-
Socioeconomic status
References
Ramachandran A, Snehalatha C, Kapur A et al (2001) High prevalence of diabetes and impaired glucose tolerance in India; National Urban Diabetes Survey. Diabetologia 44:1094–1101
Seshiah V, Balaji V, Balaji MS, Sanjeevi CB, Green A (2004) Gestational diabetes mellitus in India. J Assoc Phys India 52:707–711
Yajnik CS (2004) Early life origins of insulin resistance and type 2 diabetes in India and other Asian countries. J Nutr 134:205–210
Yajnik CS, Deshpande SS, Jackson AA et al (2008) Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune maternal nutrition study. Diabetologia 51:29–38
Thurnham DI, Bender DA, Scott J, Halsted CH (2001) Water soluble vitamins. In: Garrow JS, James WPT, Ralph A (eds) Human nutrition and dietetics. Churchill Livingstone, Edinburgh, pp 249–288
Pathak P, Kapil U, Yajnik CS, Kapoor SK, Dwivedi SN, Singh R (2007) Iron, folate, and vitamin B12 stores among pregnant women in a rural area of Haryana state, India. Food Nutr Bull 28:435–438
Srihari G, Eilander A, Muthayya S, Kurpad AV, Sheshadri S (2007) Nutritional status of affluent Indian school children: what and how much do we know? Indian Pediatr 44:204–213
Taneja S, Bhandari N, Strand NA et al (2007) Cobalamine and folate status in infants and young children in a low to middle income community in India. Am J Clin Nutr 86:1302–1309
Refsum H, Yajnik CS, Gadkari M et al (2001) Hyperhomocysteinemia and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian Indians. Am J Clin Nutr 74:233–241
Hill JC, Krishnaveni GV, Annamma I, Leary SD, Fall CHD (2005) Glucose tolerance in pregnancy in South India: relationships to neonatal anthropometry. Acta Obstet Gynecol Scand 84:159–165
Krishnaveni GV, Hill JC, Veena SR et al (2007) Gestational diabetes and the incidence of diabetes in the five years following the index pregnancy in South Indian women. Diabetes Res Clin Pract 78:398–404
Garrow JS (2000) Composition of the body. In: Garrow JS, James WPT, Ralph A (eds) Human nutrition and dietetics. Churchill Livingstone, Edinburgh, pp 13–24
Kuppuswamy B (1962) Manual of socio-economic status scale. Manasayan, Delhi
Carpenter MW, Coustan DR (1982) Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol 159:768–773
Diabetes Trials Unit. HOMA calculator. Available from www.dtu.ox.ac.uk/index.php?maindoc=/homa/, accessed 2 March 2009
Kelleher BP, Broin SD (1991) Microbiological assay for vitamin B12 performed in 96-well microtitre plates. J Clin Pathol 44:592–595
Horne DW, Patterson D (1988) Lactobacillus casei microbiological assay of folic acid derivatives in 96-well microtiter plates. Clin Chem 34:2357–2359
Clarke R, Grimle EJ, Schneede J et al (2004) Vitamin B12 and folate deficiency in later life. Age Ageing 33:34–41
World Health Organization (1999) Definitions, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO consultation. Available from www.who.int/entity/diabetes/currentpublications/en, accessed 28 January 2006
International Diabetes Federation (2006) The IDF consensus worldwide definition of the metabolic syndrome. Available from http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf, accessed 6 April 2009
Kimmons JE, Blanck HM, Tohill BC, Zhang J, Kahn LK (2006) Associations between body mass index and the prevalence of low micronutrient levels among US adults. MedGenMed 8:59
Mahabir S, Ettinger S, Johnson L et al (2008) Measures of adiposity and body fat distribution in relation to serum folate levels in postmenopausal women in a feeding study. Eur J Clin Nutr 62:644–650
Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF (2000) Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 72:690–693
Farrant HJ, Krishnaveni GV, Hill JC et al (2009) Vitamin D deficiency is common in Indian mothers but is not associated with gestational diabetes or variation in newborn size. Eur J Clin Nutr 63:646–652
Scott JM (1992) Folate-vitamin B12 interrelationships in the central nervous system. Proc Nutr Soc 51:219–224
Selhub J, Morris MS, Jacques PF (2007) In vitamin B12 deficiency, higher serum folate is associated with increased total homocysteine and methylmalonic acid concentrations. Proc Natl Acad Sci U S A 104:19995–20000
Morris MS, Jacques PF, Rosenberg IH, Selhub J (2007) Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr 85:193–200
Ocke MC, Schrijver J, Obermann-de Boer GL, Bloemberg BP, Haenen GR, Kromhout D (1995) Stability of blood (pro)vitamins during four years of storage at −20 degrees C: consequences for epidemiological research. J Clin Epidemiol 48:1077–1085
Milman N, Byg KE, Bergholt T, Eriksen L, Hvas AM (2006) Cobalamine status during normal pregnancy and postpartum: a longitudinal study comprising 406 Danish women. Eur J Haematol 76:521–525
Acknowledgements
We are grateful to the women who participated, to B. D. R. Paul, former Director; S. C. Karat, the current Director of the HMH, and the obstetrics consultants. We thank M. N. Jayakumar, S. Geetha, K. J. Chachyamma, A. Saroja, T. Gerald, G. Singh, Kiran, J. Pearce and P. Coakley for their contributions, and Sneha-India for its support. The study was funded by the Parthenon Trust, Switzerland, the Wellcome Trust, UK, and the MRC Epidemiology Resource Centre, UK. The data from this paper have been published as a conference abstract in Early Human Development (2007);83 [Suppl]:S152.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krishnaveni, G.V., Hill, J.C., Veena, S.R. et al. Low plasma vitamin B12 in pregnancy is associated with gestational ‘diabesity’ and later diabetes. Diabetologia 52, 2350–2358 (2009). https://doi.org/10.1007/s00125-009-1499-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-009-1499-0