Abstract
Background: Electronic health records (EHRs) may support randomized controlled trials (RCTs). We aimed to describe the current use and costs of EHRs in RCTs, with a focus on recruitment and outcome assessment.
Methods: This descriptive study was based on a PubMed search of RCTs published since 2000 that evaluated any medical intervention with the use of EHRs. Cost information was obtained from RCT investigators who used EHR infrastructures for recruitment or outcome measurement but did not explore EHR technology itself.
Results: We identified 189 RCTs, most of which (153 [81.0%]) were carried out in North America and were published recently (median year 2012 [interquartile range 2009–2014]). Seventeen RCTs (9.0%) involving a median of 732 (interquartile range 73–2513) patients explored interventions not related to EHRs, including quality improvement, screening programs, and collaborative care and disease management interventions. In these trials, EHRs were used for recruitment (14 [82%]) and outcome measurement (15 [88%]). Overall, in most of the trials (158 [83.6%]), the outcome (including many of the most patient-relevant clinical outcomes, from unscheduled hospital admission to death) was measured with the use of EHRs. The per-patient cost in the 17 EHR-supported trials varied from US$44 to US$2000, and total RCT costs from US$67 750 to US$5 026 000. In the remaining 172 RCTs (91.0%), EHRs were used as a modality of intervention.
Interpretation: Randomized controlled trials are frequently and increasingly conducted with the use of EHRs, but mainly as part of the intervention. In some trials, EHRs were used successfully to support recruitment and outcome assessment. Costs may be reduced once the data infrastructure is established.
See related article at www.cmaj.ca/lookup/doi/10.1503/cmaj.180841
Randomized controlled trials (RCTs) are the standard for evaluating benefits and harms of medical treatments. However, they are often time consuming and expensive to conduct, and some trials rely on strictly standardized research settings that may limit the generalizability of their results.1 Electronic health records (EHRs) — electronic databases containing patient-level variables that are gathered during routine medical care (Appendix 1, available at www.cmajopen.ca/content/7/1/E23/suppl/DC1) — provide great potential for implementing large and pragmatic trials.2,3 Randomized controlled trials could be integrated directly into routine care, offering almost perfect generalizability of their results.4 Recently, the Patient-Centered Outcomes Research Institute awarded US$332 million to 28 pragmatic clinical studies, many of them using EHR infrastructures and many of them integrated in routine care.5
Great debate regarding the potential barriers and limitations of EHR use in clinical research persists, and further details on these obstacles have been discussed elsewhere.3,6 Briefly, the 2 largest direct advantages of using routinely collected data for clinical trials may be the facilitation of patient recruitment and of outcome assessment. Randomization of treatment may occur directly from the EHR during the patient’s visit, maximizing recruitment rates.7 Recruiting patients through the EHR would allow investigators to prescreen for eligibility before approaching the potential participant, thus enabling tailoring of the efforts toward the appropriate sample; furthermore, rapid consecutive enrolment would favour recruitment through automatic screening and selection of participants through the EHR database.8 This could substantially boost trials requiring large samples or slowly recruiting trials. Yet, the ability to assess outcomes without having to measure or collect them could be the most appealing resource-sparing advantage of EHRs in RCTs. Even when funds are not at issue, just the decrease in logistical difficulties themselves, particularly in large RCTs, could be worth extracting routinely collected EHR data. Thus, the EHR may have an important role in the potential for implementing large and pragmatic trials.2,3 This offers entirely new perspectives on evaluating health care interventions that favour the development of learning health care systems.7
However, the cost associated with implementing the EHR/electronic medical record infrastructure may be substantial.9 Although one could argue that using EHRs for research purposes might lead to more affordable trials, to our knowledge, there is no systematic overview of empirical cost estimates per individual trial participant in EHR-supported RCTs. We conducted a systematic descriptive survey of the use of EHRs in RCTs to determine how EHRs are implemented in clinical research settings and to describe specifically how this technology is used to support recruitment and outcome assessment. We aimed to determine the frequency of use of EHRs and describe possible applications of EHR technology in current practice, focusing on trials that were supported by the EHR rather than evaluating the EHR itself. We also aimed to determine the cost of using EHRs for RCTs.
Methods
We performed a descriptive study assessing the current use of EHR technology in RCTs. We included any RCT in humans published in English since January 2000 that addressed any health-related topic and that used EHRs for any purpose, including participant recruitment, intervention delivery or outcome assessment.10 Focusing on modern technology, we did not include older trials. There were no other eligibility criteria.
Definitions of the EHR and related data vary.10–12 Our working definitions are shown in Appendix 1. Briefly, we considered EHRs an archive of health-related data in digital form, collected during routine clinical care for each individual patient, stored and exchanged securely and accessible by multiple authorized users in a network of care providers.11 The EHR infrastructure used in eligible RCTs must have already existed, and data must have been obtained through a query of the EHR database (i.e., no data fabricated specifically for the experiment would be considered routinely collected, for example, when the trial was about the novel implementation of an EHR v. no such implementation). There is no protocol published for this descriptive study.
Literature search
We searched PubMed (last search on Sept. 13, 2017) for English-language articles published since Jan. 1, 2000 using keywords including “electronic health record,” “electronic medical record,” “health information exchange,” “patient health record” and “e-health” with an established RCT filter13 (Appendix 2, available at www.cmajopen.ca/content/7/1/E23/suppl/DC1). Our search integrated the search strategy for EHRs provided by the United States National Library of Medicine14 and was developed with the support of an information specialist (H.E.). Two reviewers (K.A.M. and H.E. or A.L.) screened titles and abstracts. We obtained any article deemed pertinent by at least 1 reviewer as full text. One reviewer (K.A.M.) evaluated full texts and determined eligibility, and another reviewer (L.G.H) confirmed all exclusions.
Data extraction
We classified eligible RCTs based on the way in which the EHRs were used for 1) patient recruitment in any form, 2) outcome assessment in any form, 3) the trial intervention itself or 4) other possible purposes. For patient recruitment, we considered any effort of identifying trial participants based on certain characteristics that was done through an EHR query, as well as any random allocation of consecutive patients done through the EHR. For outcome assessment, we considered any trial in which any of the outcomes was obtained by querying or manually checking the EHR document (thus, where the outcome was routinely found within the EHR).
We then subclassified included RCTs into 1) EHR-supported trials, in which the EHR was used as research tool for conducting the trial (e.g., when patients with certain conditions are identified as enhanced recruitment strategy or adverse outcomes are queried through a hospital) and 2) EHR-evaluating trials, in which use of an EHR or an EHR modification was evaluated as part of the randomly allocated intervention (e.g., software alteration or addition, such as randomized implementation of a drug interaction alert system in a hospital’s EHR ordering system). Furthermore, we extracted the RCT’s research question, other study characteristics (sample size, country of origin and unit of randomization) and whether the trial included order entry systems (computerized physician order entry system or clinical decision-support system) (see Appendix 1), telehealth or personal health records.
For EHR-supported trials, we also determined the trial setting and more specific EHR uses (type of EHR and application in the trial, such as the type of alerts it would display in decision-support systems). Furthermore, we extracted whether an advanced algorithm for patient identification/recruitment or other purpose was developed. We also recorded whether the recruitment was done prospectively (e.g., by advertisement and invitation, not through the EHR), concurrently (i.e., in the point-of-care setting, through the EHR) or retrospectively (i.e., screening a patient list, through the EHR or not), and whether routinely collected data were the only outcome source or whether a hybrid approach was used. A hybrid approach could be that 1) some outcomes were based on routinely collected data alone and other outcomes were entirely actively collected or 2) some outcomes were measured based on routinely collected data, and this measurement was supplemented by active data collection (e.g., when reported by patients outside an EHR network), or a relevant amount (more than 10% of the total routinely collected data source) was manually checked for validation. We specifically recorded the primary outcome of the trial and whether it was measured with the use of routinely collected EHR data alone, when it was measured (duration of follow-up), and any information on missing data or loss to follow-up. Furthermore, we extracted, for each trial, whether blinding and allocation concealment measures were performed. We searched the full texts for keywords, such as “placebo,” “blind,” “label” and “mask,” to identify such statements and then proceeded with extracting the statement when reported. One reviewer (K.A.M.) extracted all data. We aimed to provide a general overview on potential issues of bias in the EHR-supported studies. Two reviewers (K.A.M. and B.S.) used the Cochrane risk of bias tool,15 and a third reviewer (K.A.M., H.E., B.S. or L.G.H.) verified the assessments. Any disagreement was resolved by discussion.
Trial costs
We contacted the authors of included EHR-supported trials through a standardized email to request cost information and extracted any cost information reported in the publications. We aimed to obtain a cost estimate that would allow comparison with traditional trials. Therefore, we were not interested in costs of EHR-evaluating trials. We explained to the authors that the costs of the trial could have been divided in 3 major ways:16 1) cost of the project/trial development and preparation (e.g., insurance, travel, infrastructure, consulting, sample size calculation, database set-up), 2) cost of enrolment, treatment and follow-up (e.g. per-patient costs, salary costs, patient reimbursement costs, material and/or drug costs) and 3) cost after last patient out (e.g., data-cleaning costs, analysis costs, publication costs). We aimed for only a raw cost estimate and accepted any information we could. We converted cost values to US dollars where applicable, based on the exchange rate on Nov. 1, 2017.17 We sent the data presented here to all trial authors for confirmation.
All costs are reported in US dollars.
Statistical analysis
We report results descriptively using proportions and medians with interquartile ranges. Since our study was exploratory, we did not use any statistical tests.
Ethics approval
As this article does not contain any personal medical information about any identifiable living people, ethics approval was not required.
Results
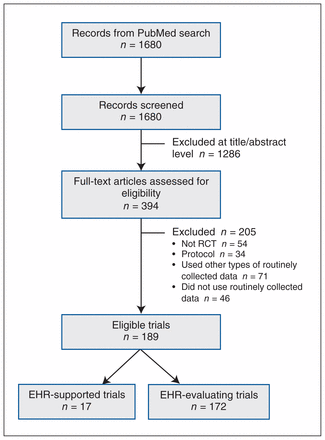
After 1680 titles and abstracts were screened, 394 potentially relevant articles were obtained as full texts, of which 189 were eligible (Figure 1). Of the 189 RCTs, 17 (9.0%) were supported by EHRs, and in 172 (91.0%), EHRs were used as a modality of intervention (EHR-evaluating).
Flow chart showing trial selection. Note: EHR = electronic health record, RCT = randomized controlled trial.
Most of both EHR-supported and EHR-evaluating trials originated from North America (13 [76%] and 140 [81.4%], respectively) and were published recently (median year 2012 [interquartile range 2009–2014]) (Table 1). Three (18%) of the EHR-supported trials and 61 (35.5%) of the EHR-evaluating trials were cluster-randomized. There were no placebo-controlled trials in our sample. The majority (101 [53.4%]) did not report the level of blinding. Blinded outcome assessment was the most frequent type of blinding (35 trials [18.5%]), followed by open label (27 [14.3%]), single-blinding (19 [10.0%]) and double-blinding (7 [3.7%]).
Characteristics of randomized controlled trials published in English between January 2000 and Sept. 13, 2017 that used electronic health records
Trials supported by electronic health records
The interventions and settings varied among the 17 EHR-supported RCTs18–34 (Table 2). Five trials (29%) used the EHR of a US Department of Veterans Affairs or affiliated facility. Most trials evaluated quality-improvement interventions, which often involved clinician education and feedback initiatives (8 [47%]), screening programs (4 [24%]), and collaborative care and disease management interventions integrated into primary care settings (3 [18%]). Almost half of the studies (8 [47%]) took place in primary care clinics, 5 (29%) were conducted in health care networks, and 3 (18%) took place in hospitals. One trial18 (6%) was performed entirely within a pharmacy electronic medical record.
Characteristics of randomized controlled trials supported by electronic health records
Supported outcome measurement
Fifteen trials (88%) measured outcomes using the EHR (Table 1). The EHR-assessed outcomes were typically screening uptake (e.g., women seeking a Papanicolaou test after receiving an automated call from the EHR prompting cervical cancer screening) (6 trials [40%]), clinical outcomes (4 [27%]), drug adherence (2 [13%]) or guideline-concordant care measures (2 [13%]). In 7 (47%) of the 15 trials, the routinely collected data source was the only source of outcome data in the entire trial. In the remaining 8 trials (53%), a hybrid approach was applied, with some outcome data being collected actively. In 419,20,28,31 of these 8 cases, the primary outcome was fully extracted from an EHR but additional outcomes were collected actively, and in 3 cases,21,22,33 the primary outcome was collected actively but additional outcomes were EHR-based. In 1 case,23 the primary outcome was collected through the EHR but was verified with actively collected data. Twelve (80%) of the 15 trials relied on the EHR for primary outcome assessment.
The median trial duration was 10 months (interquartile range 5–12 mo); in 10 (59%) of the 17 trials, the number of missing data or patients lost to follow-up was reported, but none reported on the quality of the data.
Supported recruitment
Fourteen trials (82%) used the EHR as a tool for patient recruitment (Table 1). A prospective approach was reported in 1 trial,30 and in the remaining 13 trials, the EHR was used retrospectively (i.e., manual check or simple retrospective query of eligible patients via EHR). In addition, 1 trial18 used a complex querying system (another trial27 appeared to, but this was not specifically reported). The remaining 3 trials (18%) used a (traditional) prospective recruitment approach without the use of EHRs.
Costs
We contacted 13 of the 17 corresponding authors of the EHR-supported trials. Emails were undeliverable to 3 addresses, for which we were also unable to find an alternative contact online, and we were not able to reach the authors. We obtained information on trial costs for 418,24,27,30 of the 17 trials and intervention cost data for 1 trial34 (response rate 24%).
Cost information came from 1 Australian trial18 and 4 US trials24,27,30,34 (2 within the Department of Veterans Affairs network27,34). The costs varied from $67 750 to $5 026 000 (median $86 753) for total trial costs and from $44 to $2000 (median $315) for per-patient costs (Table 3). Overall trial costs were derived from funding budgets in 3 cases,18,2,27 and 1 author stated that the overall costs were $2000 per patient.30 In the trial in which the EHR database was leveraged through automated data extraction,18 the per-patient cost was $44. In the 2 trials in which the extraction of study data from the EHR source was done manually,24,30 the per-patient cost was $560 and $2000, respectively. We have no information in this regard for 1 trial.27 For the trial that presented only the costs of the intervention (extracting data from the EHR to give feedback to health care providers),34 a cost of $44 per patient was reported when the data were extracted manually, and a sensitivity analysis indicated that this cost could decrease to $9 if the data were extracted automatically.
Costs of randomized controlled trials supported by electronic health records*
Risk of bias
Three trials had no indication for high risk of bias in any of the domains assessed18,25,26 (Table 4). There were no indications for high risk of bias related to randomization or allocation concealment in any of the trials. Most trials were open-label or assessed an intervention that was not disguisable from the participants/providers, which may indicate a high risk of bias. Relevant to EHR trials, the risk for attrition bias was generally low (missing outcome data for not more than 10% of patients), and in 4 trials, all data were reported for all patients.21,27,28,32
Risk of bias assessment with the Cochrane risk of bias tool15
Trials using electronic health records for intervention
Among the 172 EHR-evaluating trials (references in Appendix 3, available at www.cmajopen.ca/content/7/1/E23/suppl/DC1), the investigators measured outcomes using the EHR in 143 (83.1%), and the EHR was used as a tool for patient recruitment in 91 (52.9%) (Table 1). Computerized decision-support systems such as a physician order entry system or a clinical decision-support system were evaluated in 128 (74.4%). Personal health records were evaluated in 26 (15.1%). Telemonitoring-tethered devices measuring vital signs that were connected to the EHR were evaluated in 14 trials (8.1%), and electronic patient-reported outcomes were evaluated in 4 (2.3%) (Table 1).
Interpretation
In most of the identified trials in which EHRs were used, EHR technology itself was explored. However, we identified 17 trials that investigated an EHR-unrelated intervention and were supported by the use of EHRs for patient recruitment or outcome assessment. Most trials were published recently, indicating a rapid development in this field.
The potential of registry-based trials for comparative effectiveness research and the current state of using registries for RCTs, in particular for outcome ascertainment, has been reviewed recently.8,35 Interestingly, although the settings and implementation were similar to those identified in our sample, registry trials are most frequently performed in Scandinavian countries35 and EHR trials predominantly in North America. In addition, primary outcome data in registry trials are often collected with the use of routine data (82%), similarly to EHR trials (80%), which indicates confidence in the reliability of these data.35 Information about data quality and validity was rarely reported for registry-based trials (11%)35 and was not reported in any of the EHR-supported RCTs in our sample, which suggests similar reporting problems as in observational research based on routinely collected data.36 This may be expected given the current lack of a standardized reporting guideline for RCTs in which routinely collected data are used but also highlights a substantial transparency problem.
In most (84%) of the trials in our sample, outcomes were measured with the use of EHRs, including many of the most patient-relevant clinical outcomes, from unscheduled hospital admission to death. But there were also less pragmatic and more exploratory, mechanistic37,38 outcomes that help to understand pathophysiological processes: for example, in 1 study, EHR-extracted lipid levels were used during a trial of a lipid-lowering agent.39 We also identified a trial, the Salford lung study,33 that used routinely collected data in a prelicensing setting in the context of drug approval.
The identified EHR-supported trials were heterogeneous with regard to their targeted populations and outcomes measured, with a few exceptions. For example, over a third of this subsample were Veterans Affairs trials, in which the EHR was used for outcome and patient identification in all cases. This is likely due to the fact the Veterans Affairs has a long-established EHR system, and its widespread network allows for ease in designing and implementing these types of trials.
Another interesting finding that relates to the EHR-evaluating trials in our sample is the high proportion (about one-third) of trials in which cluster randomization was used. This indicates that EHR-based trials mostly evaluate interventions not at the patient level but, rather, more at a system level, as when aiming to redirect physician behaviour. This introduces the risk of contamination between the units of randomization (e.g., physicians) and thus requires a cluster design to be implemented.
Other than affordability, the great theoretical value of integrating the EHR into clinical trials lies in its potential for patient recruitment. For example, D’Avolio and colleagues40 reported on a Veterans Affairs pilot study that, like those identified in our sample, showed how convenient it can be to identify patients based on specific characteristics (the EHR database is “scanned,” and a list of possibly eligible patients results) and even to recruit them by sending an automatic electronic message to their clinician. Even with a lower response rate, when the contacted patients are in the order of thousands, this could lead to greater recruitment capacity, which could be of substantial value, particularly in RCTs in which difficult recruitment is suspected during planning. We found that, in almost half of the EHR-supported trials that used EHRs for recruitment, the investigators made use of more sophisticated techniques such as the proposed mechanisms of data mining. Although in some trials, patients were recruited by screening the EHR without specifying the use of a particular algorithm addition, most EHRs will require some programming to identify specific traits in the system that go beyond the basic EHR abilities (e.g., typing an International Statistical Classification of Diseases and Related Health Problems, 10th revision, Clinical Modification code for diabetes in a search window to obtain a list of patients, which can be done manually). More advanced EHR add-ons, which can screen for multiple variables at multiple levels simultaneously and continuously (i.e., screening the system every 2 hours or instantly during care for the entire time of the trial) require planning and validation. An example of such an EHR screening tool is one developed and used in the 2008 trial by Bereznicki and colleagues,18 in which this data-mining tool scrutinized the pharmacy electronic medical record based on a specified protocol (history of asthma medication’s being dispensed more frequently than guideline customs) to flag patients with poorly controlled asthma. These patients were then contacted, received educational material for self-management and were prompted to contact their care providers. This shows how using the EHR for patient identification and recruitment can be done efficiently yet requires substantial planning and software development. We provide a general framework with the various potential applications and challenges of using routinely collected data in different trial conduct phases elsewhere.3,6
The author-reported costs could support the assumption that using routinely collected data for RCTs may promote cost reduction as long as the outcome data source is already established and is not a financial responsibility of the research endeavour. In the 3 trials in which the EHR infrastructure was well established and was merely redirected for use in the trials,18,27,34 the cost per patient (median $44) was much lower than often-reported costs in traditional trials.41 The costs of the 2 trials in which the infrastructure was less integrated (such as actively screening the EHR for assessing the clinical outcome), a median of $1280 per patient, were more similar to those of traditional RCTs.24,30 A recently published overview of registry trials showed similar trial cost patterns (i.e., a reduction of costs when the outcome data did not require manual collection but, rather, the registry infrastructure was leveraged).8
Limitations
Some limitations of our study merit attention. First, we did not aim for a complete sample of all published EHR-based trials, and we searched PubMed only; rather, we aimed for a systematic, comprehensible and reproducible survey of the current literature. We used a highly sensitive search algorithm and implemented specific EHR search filters provided by the US National Library of Medicine. Nonetheless, we assume that we overlooked several pertinent publications that did not indicate in their keywords, title or abstract the application of EHRs. This may have engendered overrepresentation of EHRs used for interventions in our sample, and the observed disproportion of EHR-evaluating and EHR-supported trials needs to be interpreted with caution.
Second, searching for English-language articles indexed in PubMed alone may have created regional bias, with potential overrepresentation of Anglo-American studies. This could explain the high proportion of studies from the US. Nonetheless, substantial legislative and financial efforts have been made in North America to encourage the acquisition and employment of EHR technology, which may more likely be the reason for this critical imbalance.
Third, the trials were highly diverse, showing the various fields of EHR application, but we would need more data to further evaluate individual details and to explore, for example, the ethical constraints associated with no-consent point-of-care trials.42,43
Fourth, only 1 reviewer assessed the eligibility of the full texts and completed several parts of the data extraction, which may have introduced error in the selection of the trials. Nonetheless, we feel that the identified trials provide an overview of the mode of use of the EHR in RCTs.
Fifth, we did not test any hypothesis regarding the effect of using the EHR in trials, nor did we assess the impact of using the EHR on outcome ascertainment. Although we extracted a few characteristics that can point to the methodological quality of the studies, including an evaluation of major domains of risk of bias, we did not evaluate the treatment effects reported in the trials but merely offered a description of their use.
Finally, we obtained only a few rough cost estimates without details, which did not allow us to deduce any cost patterns; however, it provided first estimates to shed some light in this area.
Conclusion
Electronic health records are a novel and valuable addition to clinical research. There are numerous examples of how the EHR was implemented successfully in clinical research settings, supporting recruitment and outcome measurement in randomized trials. Electronic health records may be associated with lower research costs, allowing the conduct of more or larger RCTs. Altogether, these are promising developments toward more randomized real-world evidence.
Footnotes
Competing interests: Kimberly Mc Cord, Matthias Briel, Heiner Bucher and Lars Hemkens support the RCD for RCTs initiative, which aims to explore the use of routinely collected data for randomized clinical trials. Kimberly Mc Cord, Matthias Briel, Benjamin Speich and Lars Hemkens are members of the Making Randomized Trials More Affordable (MARTA) Group. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Lars Hemkens conceived and designed the study. Kimberly Mc Cord, Hannah Ewald and Aviv Ladanie screened titles, abstracts and full-text publications. Kimberly Mc Cord extracted the data, and Kimberly Mc Cord and Lars Hemkens analyzed the data. Kimberly Mc Cord and Lars Hemkens drafted the manuscript. All of the authors interpreted the data, critically revised the manuscript for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This work was supported by the Stiftung Institut für klinische Epidemiologie.
Disclaimer: The funder had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript or its submission for publication.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/7/1/E23/suppl/DC1.
References
- Copyright 2019, Joule Inc. or its licensors