Abstract
Background: Prognosis is difficult to establish early after moderate or severe traumatic brain injury despite representing an important concern for patients, families and medical teams. Biomarkers, such as neuron-specific enolase, have been proposed as potential early prognostic indicators. Our objective was to determine the association between neuron-specific enolase and clinical outcomes, and the prognostic value of neuron-specific enolase after a moderate or severe traumatic brain injury.
Methods: We searched MEDLINE, Embase, The Cochrane Library and Biosis Previews, and reviewed reference lists of eligible articles to identify studies. We included cohort studies and randomized controlled trials that evaluated the prognostic value of neuron-specific enolase to predict mortality or Glasgow Outcome Scale score in patients with moderate or severe traumatic brain injury. Two reviewers independently collected data. The pooled mean differences were analyzed using random-effects models. We assessed risk of bias using a customized Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool. Subgroup and sensitivity analyses were performed based on a priori hypotheses.
Results: We screened 5026 citations from which 30 studies (involving 1321 participants) met our eligibility criteria. We found a significant positive association between neuron-specific enolase serum levels and mortality (10 studies, n = 474; mean difference [MD] 18.46 µg/L, 95% confidence interval [CI] 10.81 to 26.11 µg/L; I2 = 83%) and a Glasgow Outcome Scale ≤ 3 (14 studies, n = 603; MD 17.25 µg/L, 95% CI 11.42 to 23.07 µg/L; I2 = 82%). We were unable to determine a clinical threshold value using the available patient data.
Interpretation: In patients with moderate or severe traumatic brain injury, increased neuron-specific enolase serum levels are associated with unfavourable outcomes. The optimal neuron-specific enolase threshold value to predict unfavourable prognosis remains unknown and clinical decision-making is currently not recommended until additional studies are made available.
Traumatic brain injury is the leading cause of death and disability in young adults.1,2 An important proportion of patients with severe traumatic brain injury will have a long-term or lifelong-related disability of physical, cognitive or behavioural origin.3,4 Quality of life of both the patient and his or her family can be substantially impaired.5 Therefore, early determination of prognosis is crucial for patients and clinicians.6 However, despite the availability of clinical, radiologic and electrophysiologic indicators associated with prognosis after traumatic brain injury,7-9 current prognostic indicators and models are of limited utility.10,11
Most deaths following traumatic brain injury will occur after a decision to withdraw life-sustaining therapies. These decisions are known to be variable across centres, and the process through which they are taken is not well understood.5,12 A broader multimodal scope is essential to better understand and accurately predict short-, mid- and long-term outcomes in patients with moderate and severe traumatic brain injury and assist with decision-making in the context of withdrawal of life-sustaining therapies.
The identification of tissue biomarkers as prognostic markers in patients with severe traumatic brain injury is of clinical interest11-14 and has been identified as a research priority.15 Neuron-specific enolase, an isoenzyme of the glycolytic enzyme enolase found in central and peripheral neurons,16 is one of the most studied biomarkers.14 It has been suggested as a neurologic prognostic indicator following cardiac arrest;17 however, its association with short-, mid- and long-term prognosis is unclear in patients with traumatic brain injury and is not part of standard practice.14 A recent systematic review on the topic did not identify several published studies and used a suboptimal methodology to pool data.18 Therefore, we performed a systematic review and a meta-analysis of prognostic studies to evaluate the association between neuron-specific enolase and clinical outcomes, and its prognostic value after moderate or severe traumatic brain injury.
Materials And Methods
We developed a protocol according to the guidance provided by the Cochrane Collaboration recommendations,19 and we reported results according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.20
Search strategy
We conducted a literature search using MEDLINE, Embase, The Cochrane Library and Biosis Previews (from their inception to November 2015). We used validated combinations of terms for prognostic studies providing optimal sensitivity for both MEDLINE and Embase.21,22 To maximize sensitivity, we used broad text and medical subject headings (MeSH) or Emtree terms for traumatic brain injury and biomarkers. No language restrictions were applied. Studies in languages other than English were translated as required. The full search strategy for MEDLINE is in Appendix 1 (available at www.cmajopen.ca/content/4/3/E371/suppl/DC1). Search strategies used for the other databases were adapted from our MEDLINE search strategy. We reviewed reference lists from included articles, pertinent previous narrative and systematic reviews, and we searched conference proceedings from relevant meetings (Appendix 2, available at www.cmajopen.ca/content/4/3/E371/suppl/DC1).
Study selection
Citations from the literature searches were combined, and duplicates were excluded using EndNote version X6 (Thomson Reuters). Pairs of reviewers (E.M., J.F.S., M.S., O.L. or A.B.) identified the eligible studies after independently evaluating all citations. Disagreements were resolved by an arbitrator (A.F.T.).
We included retrospective or prospective cohort studies and randomized controlled trials (RCTs) that reported data on the concentration of neuron-specific enolase sampled in the acute phase of care (i.e., care for a severe episodic or brief illness including both intensive or emergency care) after moderate (Glasgow Coma Scale score of 9-12) or severe (Glasgow Coma Scale score ≤ 8) traumatic brain injury. Our primary outcomes were mortality and either the last reported Glasgow Outcome Scale23 or Glasgow Outcome Scale-Extended score. Studies reporting 1 or more quantitative levels of neuron-specific enolase in the serum or cerebrospinal fluid and 1 of the follow-up outcome measures after discharge from the intensive care unit (ICU) were eligible. We excluded studies in which more than half of the study population were children (< 18 yr of age and for which the subgroup of adult patients could not be extracted), because the reference values for cerebrospinal fluid levels of neuron-specific enolase vary in this patient population.24 Studies involving less than 80% of patients with moderate or severe traumatic brain injury were also excluded, unless data specifically related to patients with moderate or severe traumatic brain injury could be extracted.
Data abstraction
Using a standardized abstraction form, pairs of reviewers (E.M., J.F.S., M.S., O.L. or A.B.) independently extracted data including study characteristics (i.e., country, number of centres involved, years of completion and publication and language), patient characteristics (i.e., age, gender, systemic injuries, pupil reaction, hypotension, hypoxemia and intracranial pressure measures), details of the traumatic brain injury (i.e., closed or penetrating, type of intracranial lesions, mechanism of injury and cerebral computed tomography [CT] scan results), treatments and interventions (i.e., neurosurgery, duration and type of mechanical ventilation, and ICU and/or hospital length of stay), laboratory aspects of the neuron-specific enolase testing (i.e., type of assay used, time period of sampling and sample type) and outcome evaluation (i.e., outcome definition, outcome evaluator and time period of outcome evaluation). If 2 articles reported data involving the same patient population, the article with the largest number of study participants was included unless discriminatory individual patient data were available. The furthest outcome assessment was retained when repeated measurements of outcomes were reported. We defined an unfavourable neurologic outcome as mortality, Glasgow Outcome Scale score of 3 or less, Glasgow Outcome Scale-Extended score of 4 or less. In our analyses, a Glasgow Outcome Scale score of 3 or less and a Glasgow Outcome Scale-Extended score of 4 or less were considered comparable unfavourable outcomes.
Assessment of the risk of bias
We developed a risk-of-bias assessment tool for prognostic studies based on the validated Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool, which evaluates study participation, study attrition, prognostic factor measurement, outcome measurement and confounding25 (Appendix 3, available at www.cmajopen.ca/content/4/X/E371/suppl/DC1). We used this assessment tool in a previous systematic review and meta-analysis.26
Statistical analysis
As neuron-specific enolase serum values follow a normal distribution,27 mean differences (MDs) with 95% confidence intervals (CIs) were used to evaluate the association with our primary and secondary outcomes. We resolved any uncertainties with regard to whether a study reported standard deviations or standard errors by classifying those measures according to the amplitude of the measure of central tendency in relation with the sample size and after comparison with other reported measures of variation. If uncertainty persisted, to prevent an incorrect rejection of the null hypothesis, we assumed the published statistics to be standard errors. Results were pooled using inverse-variance random-effects models.
We assessed statistical heterogeneity using the I2 statistic.28 We analyzed samples of neuron-specific enolase in serum and cerebrospinal fluid separately. Based on a priori hypotheses, we conducted subgroup and sensitivity analyses to investigate potential clinical and methodologic heterogeneity. Subgroup analyses included the time-period of outcome evaluation, sampling time, severity of traumatic brain injury, extent of associated injuries, the type of assay used and blinding. We also conducted subgroup analyses according to the risk of bias. We also conducted an a posteriori sensitivity analysis that evaluated the impact of neuron-specific enolase concentration in serum but not in plasma. We used random-effects models to generate summary estimates of mortality and Glasgow Outcome Scale scores using Review Manager version 5.0 (The Cochrane Collaboration) and SAS version 9.2 (SAS Institute Inc.). For all tests of statistical inference and CIs, we used a 2-tailed type I error rate of 5%. The quality of the evidence for the 2 main outcomes was determined using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach29 and GRADEpro software (available at http://gradepro.org).
Results
Study identification and selection
Our search strategy retrieved 5026 citations; 133 of which were reviewed in full text. Thirty unique studies30-59 (n = 1321) published between 1983 and 2015 met our eligibility criteria (Figure 1).
Flow diagram for the selection of studies. NSE = neuron-specific enolase, TBI = traumatic brain injury.
Study characteristics
Five eligible studies were published in languages other than English: 2 in Japanese (n = 89)39,51 and 3 in Chinese (n = 95).38,41,58 Twenty-nine studies were retrospective or prospective observational cohorts (n = 4-182). One study was a RCT comparing the use of hypertonic saline and isotonic saline (n = 64).31 Twenty-four studies reported serum concentrations of neuron-specific enolase (n = 1164), 1 study reported plasma concentration (n = 102) and 7 studies reported cerebrospinal fluid concentrations (n = 255).30,32,34,36,46,47,50 Three studies reported both serum and cerebrospinal fluid neuron-specific enolase concentrations.30,34,50 The earliest delay between the first traumatic brain injury and the first measurement of neuron-specific enolase obtained was as-soon-as-possible/on admission for 8 studies,31,35,38,40,43,47,52,59 up to 12 hours for 10 studies,32,33,37,39,41,42,53,55,56,58 up to 24 hours for 9 studies30,34,44,45,48-51,54 and greater than 24 hours for 2 studies.37,46 Twenty-six studies used a Glasgow Outcome Scale score of 3 or less, or a Glasgow Outcome Scale-Extended score of 4 or less to define unfavourable outcome (n = 1255), and 14 studies reported mortality (n = 700); 10 of these studies (n = 719) reported both (Table 1). Time for outcome evaluation ranged from ICU discharge up to 1 year after injury. Fifteen studies30,32,36,38,42,44,45,48-51,53-56 included patients with significant extracerebral injuries, whereas 11 studies31,33,35,37,39,40,46,47,52,58,59 included only isolated traumatic brain injury. In 4 studies, the presence of extracerebral injury was unknown.34,41,43,57 Additional characteristics of the included studies are reported in Table 1.
Risk of bias
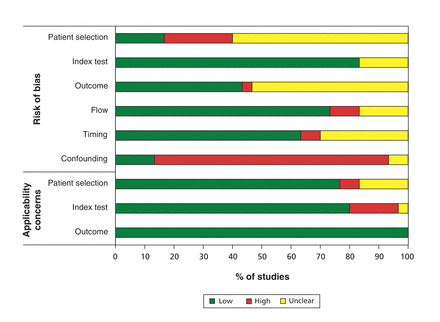
A detailed evaluation of risk of bias for the included studies is presented in Figure 2. All studies had risk of bias because none reported control for confounding; 2 studies had unclear risk of bias.41,44 More than half of the studies (16, 53%) did not report if the outcome assessors were blinded to neuron-specific enolase concentration measures. Appropriate adjustment for confounding factors was lacking in 24 studies (80%) (Figure 2). Lost to follow-up and index tests (appropriate test to evaluate neuron-specific enolase concentrations) were at low risk of bias in 22(73%) and 25 (83%) studies, respectively.
Risk of bias and applicability concerns of included studies.
Outcome measures
Blood samples
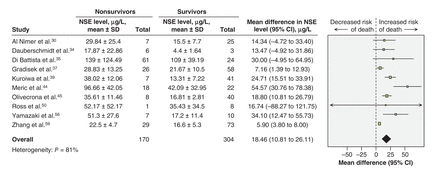
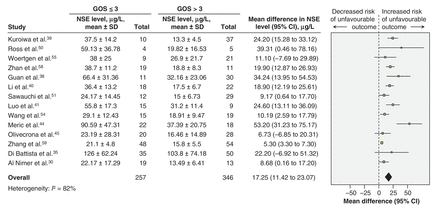
We pooled studies reporting blood concentrations (serum or plasma) of neuron-specific enolase in relation to mortality or unfavourable outcome. Many studies were excluded in the meta-analysis because they reported neuron-specific enolase concentration as median and interquartile range, peak concentration or concentration unrelated to outcome. We observed a significant association between increased blood concentrations of neuron-specific enolase and mortality (10 studies, n = 474; MD 18.46 μg/L [95% CI 10.81 to 26.11 μg/L]; I2 = 81%) (Figure 3). Increased blood neuron-specific enolase levels were also associated with a Glasgow Outcome Scale score of 3 or less [14 studies, n = 603: MD 17.25 μg/L (95%CI 11.42 to 23.07 μg/L); I2 = 82%] (Figure 4). We performed planned sensitivity and subgroup analyses (Table 2), although many could not be done because of data unavailability. Subgroup analyses evaluating biochemical analysis seemed to explain part of the observed heterogeneity. An a posteriori sensitivity analysis evaluating studies reporting neuron-specific enolase serum concentration (excluding those studies using plasma concentration) explained most of the statistical heterogeneity observed (Table 3), and more specifically when taking into consideration evaluation time, sampling time, biochemical analysis, severity of traumatic brain injury and isolated traumatic brain injuries (Table 3).
Mean differences of neuron-specific enolase blood levels in patients with moderate or severe traumatic brain injury, by mortality. A mean difference above zero indicates an increased risk of death. CI = confidence interval, IV = inverse variance, NSE = neuron-specific enolase, SD = standard deviation.
Mean differences of neuron-specific enolase blood levels in patients with moderate or severe traumatic brain injury, by neurologic outcome (defined by the Glasgow Outcome Scale Score). A mean difference above zero indicates an increased risk of poor neurologic outcome. CI = confidence interval, GOS = Glasgow outcome scale, IV = inverse variance, NSE = neuron-specific enolase, SD = standard deviation.
Discrimination thresholds
In 7 studies that presented individual data, 4 reported serum neuron-specific enolase concentrations30,42,44,50 and 6 reported cerebrospinal fluid measurements.30,32,34,46,47,50 Between-study variation in reported data made determination of an accurate threshold for poor outcome based on neuron-specific enolase value impractical. Various serum discrimination thresholds were suggested, ranging from 20 to 51.8 μg/L for mortality and 9.5 to 100 μg/L for unfavourable outcomes,32,38,40,44,45,49,53,56,58,59 and varied depending on the delay of the sampling after the traumatic brain injury and the definition of a relevant sensibility or specificity (Table 4).
Discussion
We found increased serum concentrations of neuron-specific enolase to be associated with unfavourable neurologic outcome defined as mortality or a Glasgow Outcome Scale score of 3 or less. The summary effect measures were marked by considerable heterogeneity. We could not determine threshold values associated with unfavourable prognosis.
In a systematic review that evaluated the prognostic value of neuron-specific enolase following acute ischemic stroke,60 an association between serum concentrations of neuron-specific enolase and the severity of the stroke was identified. However, the relationship between serum neuron-specific enolase concentrations and functional outcomes was unclear. In the population of patients with traumatic brain injury, studies have also reported a correlation between the presenting Glasgow Coma Scale,61 severity of parenchymal brain damage and serum concentrations of neuron-specific enolase,16,62 which provided an indication of the potential diagnostic value of neuron-specific enolase. Consistent with our results, a systematic review showed an association of serum concentrations of neuron-specific enolase with functional outcomes following cardiac arrest,17,62 although it was not as accurate as for serum concentrations of S-100β protein.17 Results of our systematic review are comparable to a previous meta-analysis on S-100β protein in which we observed significant prognostic value of S-100β measures in patients with moderate and severe traumatic brain injury, albeit neuron-specific enolase appears to be imprecise.26 Precision of neuron-specific enolase increases when biochemical assay, sample and outcome times, and patient characteristics are similar, and this should be considered in future trials.
The lack of cerebral specificity of neuron-specific enolase as compared with other biomarkers, such as the S-100β protein or glial fibrillary acidic protein, has been recently questioned63 and identified as a potential barrier to its clinical use. The serum concentration of neuron-specific enolase is known to be elevated in patients with certain types of lung cancer,64 pulmonary diseases65 and in the presence of renal failure.66 Hemolysis was also observed to increase the concentration of neuron-specific enolase in serum and cerebrospinal fluid samples67 because of its presence in erythrocytes.68 A concomitant substantial extracerebral injury could theoretically lead to an overestimation of the severity of a patient's cerebral injury and to a more somber prognosis. Nonetheless, 18 (60%) of the studies we considered in our systematic review did not exclude patients with substantial extracerebral trauma, and yet we obtained significant mean differences. Although extracerebral injuries may substantially impact serum measurements of neuron-specific enolase in patients with mild traumatic brain injury, it may not be relevant in our population of interest. Indeed, as opposed to mild traumatic brain injury, the proportion of increased serum concentration of neuron-specific enolase owing to extracerebral injuries is likely much lower and perhaps even negligible in more severe brain injuries.
A systematic review on the same topic was published while we were completing our study.18 Although the authors had comparable conclusions, we noted important limitations, including methodological flaws affecting the findings and the level of evidence. First, the search strategy was not exhaustive; we identified 11 additional publications, including 4 in languages other than English, thus reducing the possibility of a language bias. Moreover, the authors used a predetermined cut-off point to calculate sensitivity and specificity based on Glasgow Outcome Scale data from 2 studies, a cut-off that was not supported in studies evaluating mortality. In addition, the sensitivity at this cut-off point never reached 90% for the Glasgow Outcome Scale. They also assumed a right-skewed distribution of the data, but did not transform their data. In our meta-analysis, we assumed a normal distribution considering that 1 study specified the normal distribution of the neuron-specific enolase concentrations.
Cerebrospinal fluid concentrations of neuron-specific enolase are thought to more accurately reflect central nervous system damage than serum concentrations, especially in acute neurologic conditions such as encephalitis and neurocysticercosis.69 Suboptimal correlation between cerebrospinal fluid and serum concentrations of neuron-specific enolase has been observed.70 Although we did not observe an obvious difference between serum and cerebrospinal fluid samples according to reported central and dispersion measurements, data from studies having studied cerebrospinal fluid samples could not be used in pooled analyses because of the insufficient number of studies.
Strengths of our systematic review and meta-analysis include adherence to a protocol developed according to high methodologic standards. We used a tested search strategy for prognostic studies21,22 and consulted multiple databases without language restriction. This approach allowed us to be exhaustive and provide comprehensive results. Our rigorous methods were based on current guidelines for both the conduct and the reporting of systematic reviews and meta-analyses.19,20
Limitations
The strength of our conclusions is limited by the quality of included studies, which we assessed according to the reported methodological quality and risk of bias. We also observed significant statistical heterogeneity for both mortality and Glasgow Outcome Scale scores; however, owing to the limited number of studies, we could not adequately explore the sources of this heterogeneity. This is of particular importance because exploration through subgroup analyses would allow us to better understand the constraints of using neuron-specific enolase as a prognostic tool.
Conclusion
We observed a significant positive association between serum concentrations of neuron-specific enolase and unfavourable outcome (mortality or a Glasgow Outcome Scale score ≤ 3) after moderate or severe traumatic brain injury. However, we observed statistical heterogeneity that was partly explained by the type of sample and the timing of outcome assessment. Optimal neuron-specific enolase threshold values for unfavourable clinical outcomes still remain unknown. Further research must focus on understanding the optimal timing of assessment after injury and on finding accurate threshold values to inform the prediction of long-term outcome, coupled with multimodal prediction models, and assist with decisions pertaining to withdrawal of life-sustaining therapies.
Supplemental information
For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/4/3/E371/suppl/DC1
Acknowledgements
Acknowledgement: The authors thank Jean-FranÇois Simard for his help with obtaining the data.
Footnotes
Competing interests: See end of article.
Contributors: Eric Mercier, Amélie Boutin, François Lauzier, Ryan Zarychanski, Dean Fergusson, Lynne Moore, Lauralyn McIntyre, Patrick Archambault, France Légaré, François Rousseau, François Lamontage, Linda Nadeau and Alexis Turgeon contributed substantially to the conception and design of the manuscript. Eric Mercier, Amélie Boutin, Michèle Shemilt and Alexis Turgeon acquired the data. All of the authors contributed substantially to analysis and interpretation of the data, either drafted the article or revised it critically for important intellectual content, gave final approval of the version to be published and agreed to act as guarantors of the work.
References
- Copyright 2016, Joule Inc. or its licensors