Abstract
Background: There has been limited investigation of the unintended effects of routine screening for asymptomatic hypoglycemia in at-risk newborns. This study aimed to explore whether rates of exclusive breastfeeding were lower in screened babies than in unscreened babies.
Methods: This retrospective cohort study conducted in Ottawa, Canada, used data from Hôpital Montfort’s electronic health information system. Healthy singleton newborns discharged between Feb. 1, 2014, and June 30, 2018, were included. We excluded babies and mothers with conditions expected to interfere with breastfeeding (e.g., twins). We investigated the association between postnatal screening for hypoglycemia and initial exclusive breastfeeding (in the first 24 hours of life).
Results: We included 10 965 newborns; of these, 1952 (17.8%) were fully screened for hypoglycemia. Of screened newborns, 30.6% exclusively breastfed and 64.6% took both formula and breastmilk in the first 24 hours of life. Of unscreened newborns, 45.4% exclusively breastfed and 49.8% received both formula and breastmilk. The adjusted odds ratio for exclusive breastfeeding in the first 24 hours of life among newborns screened for hypoglycemia was 0.57 (95% confidence interval 0.51–0.64).
Interpretation: The association of routine newborn hypoglycemia screening with a lower initial rate of exclusive breastfeeding suggests a potential effect of screening on early breastfeeding success. Confirmation of these findings might warrant a re-evaluation of the net benefit of asymptomatic postnatal hypoglycemia screening for different newborn populations at risk of hypoglycemia.
Detectable in half of term and late preterm newborns, 1 neonatal hypoglycemia is a common problem for which management remains controversial.2,3 With a limited evidence base,1 guidelines recommend screening different populations of babies; however, the most common subpopulations at risk are newborns of birthing parents with diabetes, premature newborns, and babies small or large for gestational age.4–9 Canadian guidelines recommend screening one-third of newborns.5–9 Postnatal hypoglycemia screening involves routinely measuring newborns’ prefeed blood glucose levels to prevent adverse neurodevelopmental outcomes associated with clinically significant hypoglycemia.4 However, potential negative outcomes of screening include health care costs (e.g., blood tests),4 pain from prefeed glucose tests10 (associated with adverse neurodevelopmental outcomes),11 negative effects on the initial parent–child interaction12 and breastfeeding difficulties.13
Prefeed glucose sampling could interfere with establishing breastfeeding by delaying parental responses to feeding cues while awaiting blood tests, interposing pain with feedings, and compounding parental anxiety around feeding inadequacy.13 In a recent scoping review, 5 of 12 international guidelines cited the potential negative impacts of screening on breastfeeding,4 and only 1 primary study compared breastfeeding rates between screened and unscreened babies.14 This cohort study14 was potentially confounded by patient selection (e.g., unbalanced cesarean birth rates) and by the screened babies’ birthing parent–infant separation (both expected to reduce breastfeeding in screened babies).15 Thus, better-quality evidence is needed to understand the relation between postnatal newborn hypoglycemia screening and breastfeeding rates, which reflects the current standard of care of “rooming-in” healthy screened babies and with statistical adjustment for anticipated confounders. This study’s objective was to provide such evidence, by comparing breastfeeding rates of unscreened newborns with those of euglycemic newborns screened for hypoglycemia.
Methods
This retrospective cohort study included healthy singleton newborns delivered at Hôpital Montfort who were discharged home between Feb. 1, 2014, and June 30, 2018. Hôpital Montfort facilitates more than 3000 births annually,16 one-third of Ottawa’s 9500 annual births.17 Hôpital Montfort’s policy on newborn feeding includes creating “favourable conditions for the protection, promotion and support of breastfeeding, as described in the Baby Friendly Initiative” (Hôpital Montfort, unpublished data, 2017).
Data sources
The cohort was identified and described by searching Montfort electronic charts and the national Discharge Abstract Database (DAD). The charts and DAD were linked using chart numbers and encounter numbers. Physicians, nurses and respiratory therapists systematically entered clinical data into electronic charts. Laboratory staff entered laboratory information, pharmacy staff entered pharmaceutical data, ward clerks entered demographic data and professional coders populated the DAD by reviewing, abstracting and coding data using International Classification of Diseases codes.
Based on the study protocol’s a priori inclusion and exclusion criteria, 2 authors generated a list of 1848 potential confounder or additional exclusion variables (using Hôpital Montfort’s information-system coding). Authors M.S. (pediatrician) and V.D.Z. (obstetrician) classified the variables as potential confounders (e.g., pre-eclampsia), reasons for exclusion (e.g., HIV) or irrelevant (e.g., varicose veins). We excluded babies less likely to breastfeed owing to hypoglycemia, birth before 36 weeks’ gestation, comorbidities expected to interfere with breastfeeding (e.g., cleft palate), neonatal intensive care unit admission and serious maternal illness (e.g., shock). For the sake of parsimony, based on expertise and literature, M.S. grouped potential predictors into broader categories (Appendix 1, available at www.cmajopen.ca/content/11/2/E381/suppl/DC1). For example, “hypertension or pre-eclampsia” represented 21 database variables (Appendix 1).
Exposure
The exposure was hypoglycemia screening. From then-current Canadian Paediatric Society (CPS) guidelines5 and informal discussion with pediatricians, complete hypoglycemia screening was defined as 3 or more glucose tests, and incomplete hypoglycemia screening as 2 glucose tests.
Newborns of birthing parents with diabetes and large-for-gestational-age newborns are routinely screened for hypoglycemia in the first 12 hours of life (at 2, 5 and 11 h). Small-for-gestational-age and premature (< 37 wk gestational age) babies are screened every 3 to 6 hours in the initial 24 hours.5 Babies with suspicious clinical signs (e.g., jitters3) are assessed for hypoglycemia as well.5 Although Hôpital Montfort’s hypoglycemia protocol does not prescribe formula for a baby at risk of hypoglycemia, it notes “inadequate breastfeeding/feeds” as a risk factor for hypoglycemia (Hôpital Montfort, unpublished data, 2018).
Outcome
Our primary outcome was exclusive breastfeeding at 24 hours of life. “Exclusive breastfeeding” was defined as drinking only breastmilk, expressed or directly from the breast. Nurses routinely recorded feeding information in the newborn’s chart. Montfort pediatricians (who routinely review this recorded information when conducting rounds on newborns) expressed confidence in the reliability of nurses’ feeding documentation.
Babies discharged before 24 hours typically return home at about 6 hours of life,18 too early to describe meaningful breastfeeding rates. These newborns were excluded as “early discharges.” Most newborns remain in hospital at least 24 hours (the minimum age for newborn metabolic screening). We selected breastfeeding until a fixed age of 24 hours (rather than to discharge) to avoid confounding variables related to the duration of inpatient observation.
Statistical analysis
The estimated archival period for analysis was 4.4 years, based on 2-tailed logistic regression considering the estimated annual number of eligible newborns (2800 following a 10% exclusion rate), the estimated proportions for each screening indication (10% small for gestational age, 10% large for gestational age, 8% late preterm,7 9% with birthing parents with diabetes6), 60% rate of breastfeeding at 24 hours,19 power of 0.8 and α of 0.05 with an assumed 5% difference in breastfeeding rates (Appendix 2, available at www.cmajopen.ca/content/11/2/E381/suppl/DC1).
Descriptive statistics were computed for all study variables: totals and percentages for categorical variables, and means with standard deviations (SDs) for continuous variables.
The primary analysis compared breastfeeding rates of completely screened babies (≥ 3 glucose measures) versus other babies (unscreened or partially screened), adjusted for potential confounders. This primary analysis used a multivariable binary logistic regression analysis to model the outcome of exclusive breastfeeding at 24 hours, using the predictor variable complete glucose screening, and statistical adjustment for confounders.
A secondary multivariable logistic regression compared unscreened babies with no indication for screening with screened babies, who were subdivided by screening indication: large or small for gestational age, birthing parent with diabetes or 36-week gestational age. To account for screening indications being potential confounders, another analysis concurrently adjusted for screening and the indications for screening as 2 separate predictor variables. For this analysis, a multivariable logistic regression was performed with the base set of confounding variables added in the first step, screening indications (large or small for gestational age, birthing parent with diabetes or 36-week gestational age) in the second step and glucose screening (yes/no) in the third step. Similar approaches were used to analyze partial or complete glucose screening (≥ 2 glucose measures rather than ≥ 3) as a predictor.
Results are presented as adjusted odds ratios (ORs) with 95% confidence intervals (CIs). Complete case analysis was used for logistic regression. The choice of modelled confounders (covariates) was determined based on expertise and prior literature. Analyses were conducted in SPSS version 22 with a significance level of 5% (unadjusted).
Ethics approval
The Montfort Hospital Research Ethics Board approved the study (file no. 17-18-03-029).
Results
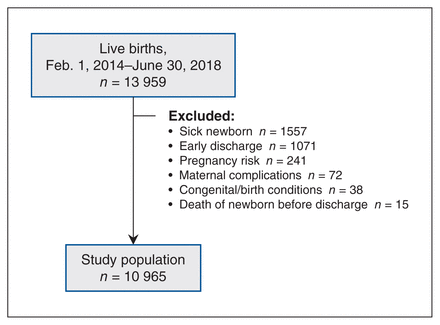
The initial study cohort included 13 959 babies. Exclusions were primarily for newborn illness (n = 1557) or discharge before 24 hours of age (n = 1071). The final sample included 10 965 newborns (Figure 1; Appendix 3, available at www.cmajopen.ca/content/11/2/E381/suppl/DC1).
Study inclusion flow diagram. Exclusion criteria were as follows: sick newborn = a newborn with an illness (e.g., transient tachypnea of the newborn requiring admission to intensive care); early discharge = discharge before 24 hours of life; pregnancy risk = a pregnancy-related condition (e.g., twin pregnancy, birth at gestational age < 35 wk); maternal complications = maternal obstetrical complications or treatment (e.g., sepsis or admission to intensive care); congenital/birth condition = newborn congenital malformations (e.g., cleft palate), global conditions (e.g., Down syndrome) or birth complications (e.g., long-bone fracture).
Complete hypoglycemia screening was recorded in 84.9% of premature newborns, 86.8% of small-for-gestational-age newborns and 76.6% of newborns of birthing parents with diabetes, and 45.8% of large-for-gestational-age newborns were completely screened (Table 1). The most common reason for complete glucose screening was large for gestational age (29.6%), followed by birthing parent with diabetes (22.0%), prematurity (6.9%) and small for gestational age (6.8%). Few unscreened babies had a recorded risk factor, most commonly being large for gestational age (7.6% of babies with < 3 recorded glucose measures). The pre- and postnatal profile of screened and unscreened babies differed regarding hypoglycemia risk factors, hypoglycemia screening, antenatal obesity and induction rates (Table 2).
Glucose measurements recorded per newborn for all babies and by screening indication
Characteristics of newborns*
Similar percentages of screened and unscreened babies were exclusively formula-fed. However, within the first 24 hours, fewer screened babies exclusively breastfed (30.6%) compared with unscreened babies (45.4%). Fifteen percent more screened newborns than unscreened newborns drank both formula and breastmilk (64.6% v. 49.8%; Table 3).
Methods of feeding used in the first 24 hours of life*
In the primary multivariable logistic regression model (Table 4), newborns completely screened with 3 or more glucose measures were less likely to exclusively breastfeed at 24 hours than newborns with fewer than 3 glucose measures, with an adjusted OR of 0.57 (95% CI 0.51–0.64). The same analysis performed with partial or complete screening (≥ 2 measurements) found the OR of exclusive breastfeeding to 24 hours of life was 0.58 (95% CI 0.52–0.64).
Multipredictor model of exclusive breastfeeding (n = 9709)*
In the secondary multivariable logistic model, each subgroup of newborns completely screened for hypoglycemia was less likely to exclusively breastfeed at 24 hours than newborns not at risk of hypoglycemia and not completely screened for hypoglycemia (Table 5). The adjusted ORs were 0.23 (95% CI 0.14–0.38) for 36-week gestational age newborns, 0.25 (95% CI 0.14–0.44) for small-for-gestational-age newborns, 0.57 (95% CI 0.44–0.73) for newborns of birthing parents with diabetes and 0.72 (95% CI 0.59–0.87) for large-for-gestational-age newborns.
Multipredictor model of exclusive breastfeeding, screening status divided by indication (n = 8297)*
In a regression analysis (Table 6) that modelled exclusive breastfeeding with separate parameters for screening (yes/no) and screening indication (e.g., premature), completely screened newborns were less likely to exclusively breastfeed at 24 hours (OR 0.64 [95% CI 0.56–0.73]).
Multipredictor model of exclusive breastfeeding, including screen status and screen indications (n = 9709)*
Interpretation
This study addresses whether hypoglycemia screening affects breastfeeding. This question is relevant to the large proportion of babies born in high-resource hospital environments where international guidelines suggest widespread postnatal hypoglycemia screening despite the uncharacterized risk to breastfeeding success.4 The cohort study by Ferris and colleagues,14 which was more limited by confounding variables, found breastfeeding to be less frequent among screened babies, resulting in more formula feeding. Likewise, CPS-guideline-informed hypoglycemia screening at Montfort was associated with a large, significant decrease in the rate of exclusive breastfeeding at 24 hours of life, regardless of the indication for screening, and independently of the indications for screening. The significant results were consistent across sensitivity analyses and with respect to the thresholds for “complete” or “partial” screening. A dose–response effect was observed, with exclusive breastfeeding less frequent among babies screened for 24 hours (babies small for gestational age or 36-weeks gestational age), compared with infants routinely screened for 12 hours (large for gestational age or with birthing parents with diabetes).
Study strengths include the large cohort and the systematically collected data entered independently from the study team. Detailed electronic hospital records facilitated consideration of many potential confounders. For example, access to point-of-care glucose test values allowed us to exclude newborns who were hypoglycemic based on the CPS criteria,5 excluding newborns whose formula intake was treatment for hypoglycemia, rather than a consequence of screening. Besides hypoglycemia-screening indications, the potential confounders appeared balanced between screened and unscreened groups, and were statistically adjusted for in the analyses.
Aside from birth year and postnatal continuous positive airway pressure (CPAP), associations between breastfeeding and predictors were plausible, and expected. We did not have expectations regarding the birth-year predictor, and the estimate for postnatal CPAP was smaller and in the opposite direction to a high Apgar score; overall, postnatal distress appeared negatively correlated to exclusive breastfeeding, as expected. The model parameters’ plausibility supports the models’ face validity.
Although our findings date back to 2014, they appear relevant. The 2004 hypoglycemia screening guidelines5 were updated in 2019,20 outside of the study period, and with no modifications to the screening or treatment of at-risk normoglycemic babies. Similarly, other changes such as limited human resources and visitors access in the post-COVID-19 hospital environment are not expected to attenuate the reported findings.
Replication of this study’s findings is warranted. Validation through replication would support theoretical concerns about the risks of expanding screening and suggest efforts to mitigate its unintended effects. Ideally, future research would balance the intended neurodevelopmental benefits of hypoglycemia screening against unintended effects (on rates of beneficial breastfeeding,26,27 and harms from newborn pain and parental stress). It would be informative to conduct a randomized trial of neurodevelopmental outcomes with and without hypoglycemia screening.28 Such investigations may begin with the most controversial or borderline screening indications, as overscreening may cause overuse of formula supplementation, among other problems. Such research could affect life’s beginnings for many people born in high-resource, inpatient settings worldwide.
Limitations
Limitations of this study include generalizability, the potential to confound the effects of screening with the indications for screening, the frequent observation of hypoglycemia screening of newborns without a study-documented indication (35% of screened babies lacking an identified risk factor, suggesting database limitations), and the amount of missing data (12% for primary and tertiary logistic regressions and 24% for secondary logistic regression).
The findings of this single-centre study may not apply to other centres, a possibility warranting replication elsewhere. Also, exclusive breastfeeding in the first 24 hours is a surrogate for long-term establishment of breastfeeding. It is a recognized end point, however. Recognizing the association between the immediate postnatal period and long-term breastfeeding outcomes, the World Health Organization and UNICEF implemented their Baby Friendly Hospital Initiative21 decades ago, with 9 of their 10 instructions bearing on infant feeding in the immediate postnatal period. Exclusive breastfeeding post-natally in hospital is associated with exclusive breastfeeding at 1 month of age and beyond.22–24 Therefore, it would be worth replicating this study with longer-term feeding outcomes.
Although all the models found independent negative associations between hypoglycemia screening and breastfeeding rates, there were also associations between screening-population identity and breastfeeding rates (with lower rates in preterm and small-for-gestational-age babies [Tables 5 and 6]). These latter associations confound the relation of screening with breastfeeding.
Newborn hypoglycemia screening may not have corresponded to the basic documented screening indications for several reasons. Some babies exhibited potential symptoms (e.g., jitters), which appropriately led to testing for hypoglycemia; however, a euglycemic baby without preidentified risk factors would rarely be tested for hypoglycemia 3 or more times. A subgroup of this study may have demonstrated protocol nonadherence; for example, perhaps babies large for gestational age were screened less frequently because they are more difficult to identify. Parents may decline screening. Finally, there could have been an issue with data quality, perhaps in the misclassification of hypoglycemia screening or of indications for screening (e.g., a rate of birthing parent diabetes greater than the study-observed 5% was expected4).
The DAD showed excellent agreement for demographic and obstetric variables (e.g., κ typically > 0.9 for childbirth-related procedures, such as cesarian delivery, induction and vacuum traction).25 Juurlink and colleagues observed 80% to 100% agreement for newborn jaundice, prematurity, pneumothorax and respiratory distress of newborns.25 However, agreement was lower for more complex cases, and direct validation of most of this study’s variables was not found.25
Misclassification would likely bias to the null for most of the analyses; however, in the tertiary model (with separate parameters for screening status and screening indications), misclassification of screening status and of hypoglycemia risk factors could have confounded modelled estimates. As hypoglycemia treatment decreases exclusive breastfeeding, missed cases of hypoglycemia (e.g., owing to missing data) could have overestimated the effect of screening. Missing data likely occurred completely at random owing to data-entry errors or administrative reasons rather than factors related to exposure or outcome.
Conclusion
The large, significant and consistently observed decreases in the initial rate of exclusive breastfeeding with hypoglycemia screening inform an understudied yet commonly expressed concern raised by international postnatal hypoglycemia-screening guidelines. These results suggest that hypoglycemia screening may have unintended consequences on exclusive breastfeeding rates, highlighting the need to provide appropriate support to breastfeeding parents.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Michael Saginur contributed to conceptualization, methodology, writing (all steps), supervision, administration and funding acquisition. Joseph Abdulnour contributed to software, validation, formal analysis, data curation and writing (all steps). Eva Guérin and El Mostafa Bouattane contributed to methodology, software, resources and data curation. Xaand Bancroft contributed to software, validation, formal analysis and data curation. Daniel Corsi contributed to methodology. Vincent Della Zazzera contributed to data curation. All of the authors revised the manuscript, gave final approval for the version to be published and agreed to act as guarantors of the work.
Funding: The study was supported by a grant from the Innovation Fund Provincial Oversight Committee, Ontario (project code: AHM-18-001); however, this article’s analyses and statements are solely the authors’.
Data sharing: Data used in this article were derived from administrative health data. The data were provided under specific data-sharing agreements only for approved use at Hôpital Montfort. The original source data are not owned by the researchers and, as such, cannot be provided to a public repository. Where necessary, source data specific to this article or project may be reviewed at the Hôpital Montfort with the consent of the hospital and the required privacy and ethical review bodies.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/11/2/E381/suppl/DC1.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/
References
- © 2023 CMA Impact Inc. or its licensors