Abstract
Background: Although prophylaxis for ophthalmia neonatorum at birth is required by law in Ontario, declining prevalence of disease and efficacy of prophylaxis have called this practice into question. The objective of this modelling study was to assess the cost-effectiveness of universal prophylaxis for ophthalmia neonatorum to inform decision-makers on the potential impact of a change in this policy.
Methods: We compared the cost-effectiveness of prophylaxis for ophthalmia neonatorum with no prophylaxis through cost–utility analysis with a lifetime time horizon, considering a provincial government payer, for a hypothetical population of newborns in Ontario. We assessed both the mean incremental costs of prophylaxis and its mean incremental effectiveness using a hybrid (part decision tree, part Markov) model. We used a scenario analysis to evaluate alternative time horizons and discount rates. We conducted a threshold analysis to evaluate the impact of variations in the cost of prophylaxis and in the prevalence of sexually transmitted infections (gonorrhea and chlamydia).
Results: In our model, prophylaxis for ophthalmia neonatorum did not meet a willingness-to-pay threshold of Can$50 000 per quality-adjusted life-year (QALY). Although prophylaxis was effective in reducing morbidity associated with ophthalmia neonatorum, the number needed to treat to prevent 1 case of ophthalmia neonatorum blindness was 500 000, with an associated cost of more than Can$4 000 000. When compared with no prophylaxis, prophylaxis had an incremental cost of Can$355 798 per long-term QALY gained (incremental cost-effectiveness ratio).
Interpretation: We found that prophylaxis for ophthalmia neonatorum, although individually inexpensive, leads to very high costs on a population level. These findings contribute to the discussion on mandatory prophylaxis currently underway in several jurisdictions.
Prophylactic treatment of ophthalmia neonatorum with topical erythromycin at birth is required by law to be applied to all newborns in Ontario.1 Ophthalmia neonatorum is conjunctivitis that occurs in the first 4 weeks of life. Mandatory prophylaxis to prevent ophthalmia neonatorum has been in place since the preantibiotic era when it was predominantly caused by transmission of Neisseria gonorrhoeae, leading to rapidly progressive eye infection and potential blindness.2,3 Since the advent of antibiotic treatment for N. gonorrhoeae, coupled with universal prenatal screening, rates of ophthalmia neonatorum have declined significantly.3 Further, N. gonorrhoeae is no longer the leading cause of ophthalmia neonatorum, accounting for less than 1% of all cases in the United States.4 More common pathogens include Chlamydia trachomatis, Staphylococcus, Streptococcus and Hemophilus species.4 Erythromycin ointment does not effectively prevent these infections. The efficacy of erythromycin for ophthalmia neonatorum prophylaxis may also be declining owing to emerging resistance.5
Several high-income countries and some Canadian provinces have repealed requirements for prophylaxis of ophthalmia neonatorum.3,4 Legal mechanisms now exist for low-risk parents to opt out of prophylaxis in Ontario.1 A rapid response report from the Canadian Agency for Drugs and Technologies in Health (CADTH) found that evidence for or against universal screening is limited and evidence-based guidelines make conflicting recommendations.6 The Canadian Pediatric Society (CPS) advocates for a focus on prenatal maternal screening for certain sexually transmitted infection (STIs), namely N. gonorrhoeae and C. trachomatis, rather than universal ophthalmic prophylaxis for the newborn.4
In this modelling study, we sought to quantify the potential impact of a policy change regarding infant eye prophylaxis on quality of life. Although erythromycin ointment is an inexpensive intervention, the cost-effectiveness of its universal application should be evaluated. This may assist the government of Ontario in choosing if it will continue to require and fund universal prophylaxis for ophthalmia neonatorum within provincial hospitals, or if it will move toward relying on prenatal screening and treatment for prevention of ophthalmia neonatorum, as the CPS suggests.4
Methods
Study design
The decision problem addressed by this analysis is whether a provincial government, as a funder of health care, should fund prophylaxis for ophthalmia neonatorum. We compared the current prophylaxis regimen (base case) with no prophylaxis (alternate case) using a cost–utility analysis with a lifetime time horizon to assess both the mean lifetime incremental costs of prophylaxis for ophthalmia neonatorum and its mean incremental effectiveness. Effectiveness was expressed primarily by lifetime quality-adjusted life-years (QALYs) and with cases of blindness averted through prophylaxis and short-term QALYs. We assessed cost-effectiveness using the incremental cost-effectiveness ratio (ICER). We conducted the study from the perspective of a provincial health care payer within the single payer, Canadian health care context. We used the lifetime time horizon to capture lifetime costs of disutility through blindness from ophthalmia neonatorum. Reporting of this study was conducted using the Consolidated Health Economic Evaluation Reporting Standards for reporting of economics evaluation studies.7
Study population
The patient population is the whole population of newborns in Canada. We modelled maternal input data from pregnant people in Canada to identify neonatal exposure parameters. We did not exclude any subgroups from the modelled population.
Treatment comparators
We compared the intervention of applying 0.5% erythromycin ointment to the eyes of newborns as prophylaxis against ophthalmia neonatorum with no prophylaxis. We assessed both the intervention (current universal prophylaxis practice) and the alternative (no prophylaxis) in the context of current prenatal screening and treatment guidelines, which include the treatment of chlamydia and gonorrhea detected among pregnant people, as well as among infants found to have been exposed at the time of birth.4,8,9
Decision analysis model
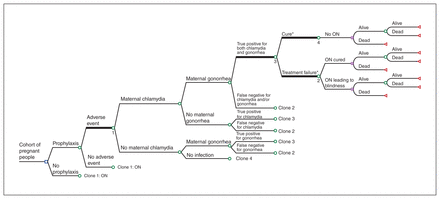
We conducted our analysis using a hybrid (part decision tree, part Markov) model constructed in Microsoft Excel 16.26 (Figure 1). The short-term decision tree model first allows determination of the proportion of mothers with or without chlamydia, with or without gonorrhea, and the proportion of newborns who have adverse events with prophylaxis. The tree then simulates the impact of STI screening and treatment, which facilitates estimation of the proportion of babies who will develop ophthalmia neonatorum and their outcome in terms of who will develop blindness. The long-term Markov model then simulates the lifetime QALYs for infants who have blindness and those who do not with a 1-year cycle and a lifetime horizon (up to age 110 yr), with the states of alive and dead.
Simplified representation of hybrid model. The thick lines indicate the nodes from which clones are specified. Note: ON = ophthalmia neonatorum, STI = sexually transmitted infection. *Cure or treatment failure refers to treatment of maternal STI and therefore probability of newborn exposure.
We modelled the risk of ophthalmia neonatorum in a hypothetical population of newborns, born to mothers who had undergone prenatal STI screening according to current Canadian guidelines (Appendix 1, available at www.cmajopen.ca/content/11/1/E33/suppl/DC1)8 and who did (base case) or did not (alternate case) receive 0.5% erythromycin ointment as prophylaxis for ophthalmia neonatorum at birth. Prenatal screening and obstetrical testing are assumed to modify baseline population risk of ophthalmia neonatorum in the same way, regardless of ophthalmia neonatorum prophylaxis. Model parameters are described in Table 1.
Model parameters
We calculated the baseline population risk of ophthalmia neonatorum based on a prevalence of 1.9% for chlamydia and 0.2% for gonorrhea in the population of pregnant females in Canada, as reported in a study that examined characteristics of females delivering in a Montréal hospital,8,10 assuming a 40% probability of gonorrhea transmission4 and a 15% probability of chlamydia transmission from mother to newborn that causes ophthalmia neonatorum.11 The effectiveness of prophylaxis in decreasing this risk is calculated based on studies that compared prophylaxis to no prophylaxis, with a relative risk of gonococcal ophthalmia neonatorum of 0.19 and a relative risk of chlamydial ophthalmia neonatorum of 0.93.12,13
Costs
We calculated costs within the model for neonatal conjunctivitis treatment, erythromycin ointment and medical assessment of adverse events related to chemical conjunctivitis from prophylaxis. We derived costs from the published literature and presented them in Canadian dollars (Appendix 2, available at www.cmajopen.ca/content/11/1/E33/suppl/DC1). For physician costs, we used the applicable current fee schedule for Ontario.14 For costs of treatment, we used published acquisition prices. We calculated the costs of missed ophthalmia neonatorum infection and subsequent blindness based on an expected lifetime of 82 years,15 discounted at a rate of 1.5% per annum.16 Full details of the costing methodology are provided in Appendix 2.
Utilities
We determined the baseline health utility of a healthy term infant from a meta-regression of pediatric HUI3 health utilities.15 We obtained long-term utility values by age, assuming average health status, from the 2014 Canadian Community Health Survey.16 Given that ophthalmia neonatorum and its treatment are not expected to have an impact on gestational age and vice versa, we assumed all infants to be born healthy at term; we did not model differential baseline health utilities of preterm and extremely preterm infants. We considered disutility from ophthalmia neonatorum that is treated and cured near the time of birth, as well as from adverse effects of prophylactic erythromycin ointment to be negligible on a lifetime time horizon. We determined the annual disutility associated with blindness from missed ophthalmia neonatorum from the same 2017 metaregression for the pediatric age group (up to age 16 yr).15 We took the disutility associated with blindness over the age of 16 years from a recent study.17
Statistical analysis
Base analysis
We obtained estimates of the expected values for outcomes and costs through a Monte Carlo simulation, with input parameters assigned a probability distribution based on their expected value and degree of uncertainty. We estimated expected values based on 10 000 replications, which was sufficient to obtain stable estimates of the costs and QALYs for both prophylaxis and no prophylaxis. We presented the underlying uncertainty around the cost-effectiveness of prophylaxis by a cost-effectiveness acceptability curve, illustrating the probability that prophylaxis is cost-effective based on different thresholds for a decision-maker’s willingness to pay for a QALY.
Scenario analysis
Scenario analyses addressed methodological and structural uncertainty. We reran our analyses, adopting alternative discount rates (0% and 3%) and different time horizons (1 yr, 10 yr and 20 yr).
In addition, we conducted 2 further threshold analyses. The first was a simple threshold analysis to identify the cost of prophylaxis, using a willingness-to-pay threshold of $50 000 per QALY for ophthalmia neonatorum prophylaxis. A further threshold analysis focused on determining the threshold of STI prevalence in the pregnant population, or a subgroup thereof, which would likewise be associated with a threshold of $50 000 per QALY.
Value of information analysis
We conducted a value of information analysis whereby we estimated the expected value of perfect partial information (EVPPI) for individual parameters and parameter groups using the Sheffield Accelerated Value of Information approach.18 This provides a basis for the value of further research and allows identification of the relative importance of parameters with respect to the risk and consequences of basing a reimbursement decision on the current level of information. For the value of information analysis, we adopted a threshold of $50 000 per QALY.
Ethics approval
Ethics approval was not required for this study.
Results
Base case
Prophylaxis was more effective than no prophylaxis in terms of both short- and long-term QALYs, and prevented cases of blindness (Table 2). However, the gain in long-term QALYs was 0.00002, and analysis suggested that the number needed to treat with prophylaxis to prevent 1 case of blindness was more than 500 000. Prophylaxis was associated with higher lifetime costs ($7.73 v. $0.75 per patient). The cost increase was primarily owing to the cost of prophylaxis plus treatment of adverse events, which were only partially offset by the savings through reduced infections.
Disaggregated results from base case analysis comparing prophylaxis for ophthalmia neonatorum with no prophylaxis
When compared with no prophylaxis, prophylaxis had an ICER per long-term QALY gained of Can$355 798 (Table 3). For analysis restricted to short-term QALY gains, the ICER was Can$12.3 million. Thus, prophylaxis would be cost-effective only if a decision-maker’s willingness to pay was greater than Can$355 798 per QALY.
Overall results of base analysis comparing prophylaxis for ophthalmia neonatorum with no prophylaxis
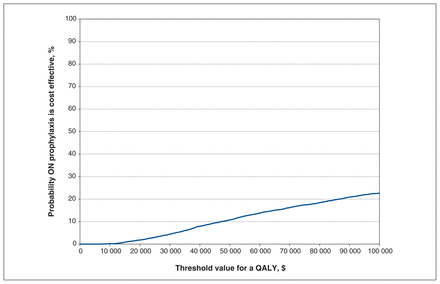
Figure 2 presents the probability that prophylaxis is optimal based on the threshold value for a QALY ranging from Can$0–$100 000. For a threshold value of $50 000 per QALY, the probability that prophylaxis is optimal is 10.7%; for a threshold of $100 000, the probability is 21.7%.
Cost-effectiveness acceptability curve. Note: ON = ophthalmia neonatorum, QALY = quality-adjusted life-year.
Scenario analysis
In all scenario analyses, prophylaxis for ophthalmia neonatorum was associated with greater costs and greater QALYs, with the ICER ranging from $207 801 to $12.3 million (Table 4). Thus, the conclusion to be drawn from each analysis did not differ from the base analysis.
Results of scenario analyses comparing prophylaxis for ophthalmia neonatorum with no prophylaxis
Based on a threshold of Can$50 000 per QALY, there was no cost associated with prophylaxis for ophthalmia neonatorum whereby prophylaxis could be considered cost-effective; at a cost of $0, the ICER for prophylaxis versus no prophylaxis was Can$163 885. The prevalence of chlamydia and gonorrhea infections would need to be 5.5 times higher than in our base analysis for prophylaxis to be considered cost-effective.
Value of information analysis
The value of information analysis found that only 2 individual parameters had nonzero EVPPI, meaning that the uncertainty around other parameters was not sufficient to change the decision with respect to whether prophylaxis for ophthalmia neonatorum is cost-effective (Table 5). The 2 parameters identified were the relative reduction in transmission with prophylaxis with prevalent chlamydia and the false-negative rate for prenatal STI testing.
Results of value of information analysis
When parameters were grouped, the value of information analysis found further research relating to 2 groups of parameters may have information value, namely the accuracy of prenatal STI testing, and the benefits (reduction in incidence of ophthalmia neonatorum) and risks (adverse events) from prophylaxis for ophthalmia neonatorum (Table 5).
Interpretation
In this study, we evaluated the cost-effectiveness of universal application of erythromycin ointment as prophylaxis against ophthalmia neonatorum in Canada in a wide range of scenarios with varied risk stratification for the target group (e.g., possible maternal STI, including false-positive and false-negative results, chlamydia coinfection) and a number of model inputs. The historical impact of prophylaxis for ophthalmia neonatorum highlights the benefits of population-based preventive interventions.2 However, in light of declining effectiveness and changing epidemiology,4 there is reason to explore the value gained by Canadian society from such an intervention within our current health system.
In our model, prophylaxis for ophthalmia neonatorum did not meet a willingness-to-pay threshold of Can$50 000 per QALY. Although prophylaxis was effective in reducing short-and long-term morbidity associated with ophthalmia neonatorum, the number needed to treat to prevent 1 case of ophthalmia neonatorum blindness was 500 000, with an associated cost of more than Can$4 000 000.
Our findings suggest that erythromycin eye prophylaxis, although individually inexpensive, leads to very high costs on a population level. For prophylaxis for ophthalmia neonatorum to be considered cost-effective, the prevalence of gonorrhea or chlamydia infections in pregnancy must be significantly higher. Although prophylaxis is not cost-effective at a population-level in Ontario, some subgroups may benefit from this intervention.
Our value of information analysis highlights how changes in the efficacy of prophylaxis and the accuracy of prenatal STI screening could affect the results. Topical prophylaxis alone will not prevent ophthalmia neonatorum caused by chlamydia. With emerging erythromycin resistance, the efficacy of topical prophylaxis against ophthalmia neonatorum caused by gonorrhea is unlikely to improve. However, future research should be undertaken if prophylaxis with other agents becomes commonplace. The accuracy of STI screening is dependent on universal access to STI testing and treatment, as well as rigorous application of the prenatal screening guideline, including identification and follow-up of high-risk patients.9 Our analysis highlights the importance of these system-level and individual-level factors, and that prophylaxis may be more cost-effective in settings with poor access to care and follow-up.
Although national guidelines in countries such as the United Kingdom, Denmark and Sweden have recommended stopping universal prophylaxis of ophthalmia neonatorum,19 the practice continues in the US and in many Canadian provinces.6 Current literature on the subject of the cost-effectiveness of prophylaxis for ophthalmia neonatorum is limited to comparisons between types of prophylaxis, rather than the cost-effectiveness of prophylaxis itself.6
Our findings are based on theoretical outcomes and may overestimate rates of ophthalmia neonatorum–related blindness. For example, no cases of ophthalmia neonatorum–related blindness have been reported in the UK as of the 25-year follow-up after abandoning prophylaxis.20 In addition, a recent Cochrane meta-analysis evaluating rates of gonococcal conjunctivitis in infants treated with prophylaxis or placebo indicated that the studies reviewed did not report on blindness as an outcome, and that the effect of prophylaxis on ophthalmia neonatorum had low- to very low–certainty of evidence.21
Limitations
Limited data are available on the real-world effectiveness of erythromycin ointment in the prevention of ophthalmia neonatorum. The effectiveness parameter used here came from a study that pooled data from use of silver nitrate and erythromycin ointment and, therefore, could overestimate the effectiveness of erythromycin alone. We also did not factor antibiotic resistance to erythromycin ointment into our calculation. With rates of gonococcal resistance to erythromycin of up to 30% in some regions, antimicrobial resistance may reduce the efficacy of prophylaxis.5 This would, consequently, further reduce the cost-effectiveness of prophylaxis and would not change our conclusions. In addition, we modelled our study on the presumption that treatment failure or lack of treatment may lead to blindness, but little data exist on ophthalmia neonatorum–related blindness in developed countries in recent years.20,21 Our results may, therefore, overestimate the cost-effectiveness of prophylaxis for ophthalmia neonatorum. When costing the adverse effects associated with prophylaxis, we assumed that these would be assessed in an outpatient setting, given their mild nature and time course.22 However, this costing may be an overestimate should the chemical conjunctivitis be assessed before discharge from hospital or during another routine appointment. We included a model for cost of treatment of ophthalmia neonatorum caused by chlamydia, given its inclusion in CADTH definitions of ophthalmia neonatorum. However, despite small effects reported in the literature, erythromycin prophylaxis is generally not considered clinically effective in preventing ophthalmia neonatorum caused by chlamydia, which might reduce the cost value of erythromycin prophylaxis even further.
Conclusion
Our findings suggest that, given a willingness-to-pay threshold of Can$50 000 per QALY, prophylaxis against ophthalmia neonatorum with erythromycin ointment is not cost-effective in Canada, particularly in the context of the low prevalence of gonococcal infection among pregnant people. Although prophylaxis is an inexpensive intervention on an individual level, when applied to a population level, it becomes very costly. These findings are consistent with the current CPS recommendations regarding universal eye prophylaxis. There remains a need to study the efficacy of erythromycin ophthalmic prophylaxis in view of current resistance patterns.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Ellen Rowlands Snyder conceived the study; all of the authors contributed to its design, and to the acquisition, analysis and interpretation of data. All of the authors drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Data sharing: All data used within the model were derived from public sources. All data are provided in the manuscript to allow model replication.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/11/1/E33/suppl/DC1.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/
References
- © 2023 CMA Impact Inc. or its licensors