Abstract
Background: Several vaccines against SARS-CoV-2 have been developed and approved at an unparalleled speed. Given that SARS-CoV-2 vaccines are recommended to pregnant people, our aim was to quantify vaccination uptake, and describe vaccination hesitancy and behavioural attitudes surrounding SARS-CoV-2 vaccination in pregnancy in Canada.
Methods: The CONCEPTION study is an ongoing international study started in June 2020, evaluating the impact of the COVID-19 pandemic on the health of pregnant people and their children. For this study, pregnant people recruited from Apr. 20, 2021, to Feb. 8, 2022, and residing in Canada were invited to complete a Web-based survey. In addition to all CONCEPTION variables, data on vaccine uptake as well as personal knowledge of COVID-19 severity in pregnancy and of SARS-CoV-2 vaccine safety and efficacy were collected. Marginal risk differences and adjusted odds ratios (ORs) were calculated to assess determinants of SARS-CoV-2 vaccination during pregnancy.
Results: From Apr. 20, 2021, to Feb. 8, 2022, 603 pregnant people were recruited and gave consent, of which 83.7% (n = 505) were vaccinated and 16.3% (n = 98) were not vaccinated against SARS-CoV-2. Uptake of the influenza vaccine in 2020/21 was a significant predictor of being vaccinated against SARS-CoV-2 or intention to be vaccinated (marginal risk difference 3.2%, 95% confidence interval [CI] 3.0% to 3.3%, adjusted OR 4.43, 95% CI 2.32 to 9.25), and being employed (marginal risk difference 11.2%, 95% CI 10.6% to 11.9%, adjusted OR 2.17, 95% CI 1.03 to 4.35) increased the likelihood of being vaccinated against SARS-CoV-2. Self-assessed knowledge of COVID-19 severity and vaccine efficacy was not associated with vaccine uptake.
Interpretation: Among the Canadian pregnant people who responded to this study, vaccine uptake against SARS-CoV-2 was high. However, our results underscore the importance of improving knowledge transfer about the efficacy of SARS-CoV-2 vaccines in pregnancy to guide vaccination efforts.
Several SARS-CoV-2 vaccines have been developed and approved at an unparalleled speed, while maintaining rigorous regulatory processes.1–4 At the start of our study period in April 2021, there were limited data available on the efficacy of SARS-CoV-2 vaccines in pregnancy, mostly owing to the fact that pregnant people were excluded from preauthorization clinical trials, and only limited human data on safety during pregnancy were available at the time of authorization. However, we know that pregnant people with COVID-19 are at increased risk for severe illness (e.g., resulting in admission to an intensive care unit, extracorporeal membrane oxygenation or mechanical ventilation) and death, as compared with nonpregnant people of reproductive age.5,6 Following the Pregnancy Research Ethics for Vaccines, Epidemics, and New Technologies (PREVENT) working group recommendations,5 both the Society for Maternal-Fetal Medicine and other women’s health organizations have included pregnancy as a risk factor for severe COVID-19.
Thus far, the SARS-CoV-2 vaccines appear to be equally effective in pregnant and nonpregnant people.7 Indeed, data from developmental and reproductive toxicity animal-model studies for the Pfizer-BioNtech, Moderna and Janssen (Johnson & Johnson) SARS-CoV-2 vaccines did not demonstrate any safety concerns in pregnancy.7,8 Moreover, in a sample of pregnant people in the United Kingdom admitted to hospital for COVID-19-related symptoms during the wildtype, Alpha and Delta dominance periods, efficacy data of the Pfizer-BioNtech and Moderna vaccines showed that no fully vaccinated pregnant people were admitted to intensive care units between Feb. 1, 2021 (when vaccination data collection commenced) and Nov. 7, 2021.9
On Apr. 20, 2021, the Society of Obstetricians and Gynaecologists of Canada (SOGC) declared supporting the use of all available SARS-CoV-2 vaccines approved in Canada in any trimester of pregnancy.10 Recommendations for vaccination against SARS-CoV-2 in pregnant people were further revised and approved on May 13, 2021, by the Public Health Agency of Canada,11 and the SOGC statement related to vaccination in Canada was later updated on May 25, 2021, approving SARS-CoV-2 vaccination in pregnant people.8 The Comité sur l’immunisation du Québec later recommended that the mRNA vaccines, such as those from Moderna or Pfizer-BioNtech, should be preferred over non-mRNA vaccines for pregnant people as there are more safety data with these vaccines during pregnancy.12
Data on SARS-CoV-2 vaccine acceptance among pregnant people are critical, as vaccine hesitancy is a major threat to global health, as described by the World Health Organization (WHO).13 Vaccine acceptance has been shown to depend on several factors, such as sociodemographic characteristics, confidence in vaccine safety and efficacy, available information on disease severity, and trust in the health system and health care providers.8,14–17 Additionally, the KFF (Kaiser Family Foundation) COVID-19 Vaccine Monitor report noted in December 2020 that about 27% of a random sample of 1676 American adults would probably not or definitely would not get a SARS-CoV-2 vaccine if available.18 In July 2021, the 2 age brackets with the lowest vaccination rates among Canadian adult females were 18–29 years and 30–39 years (73% and 76% of 1-dose vaccination, respectively),19 which would include most people of childbearing age.
In the context of this pandemic, we aimed to quantify vaccine uptake in pregnancy and describe the level of and reasons for hesitancy and behavioural attitudes surrounding the SARS-CoV-2 vaccine among pregnant people in Canada. We hypothesized that influenza vaccine uptake in the previous flu season, socioeconomic status and education level would be predictors of SARS-CoV-2 vaccine acceptance and uptake.20
Methods
The CONCEPTION cohort started on June 26, 2020, and is ongoing.21,22 The CONCEPTION study is an international study (Canada, China, the United States and France being among the most represented), which evaluates the impact of the COVID-19 pandemic on maternal mental health in pregnancy and its impact on their children, with long-term follow-up on both mothers and children. Since Apr. 20, 2021, CONCEPTION has collected data on vaccine acceptability, uptake prevalence, adverse effects and vaccine hesitancy. This date was chosen as it coincides with public messaging from the SOGC and Canadian public health agencies, as well as the time when pregnant people were considered to be a prioritized group for the vaccination campaigns. The study obtains patient consent and collects data online using SurveyMonkey (Appendix 1, available at www.cmajopen.ca/content/10/4/E1034/suppl/DC1), which is a secure platform that enabled recruitment worldwide, and facilitates the validation of double entry and participation by deleting questionnaires filled using the same IP address.
Eligibility criteria
Study eligibility assessment, consent and baseline data collection are completed electronically; the information is thereafter downloaded on a secure server at CHU Sainte-Justine, Montréal. All data collected are in a centralized database at CHU Sainte-Justine. For this study on SARS-CoV-2 vaccination, we included CONCEPTION study participants between Apr. 20, 2021, and Feb. 8, 2022. Participants were pregnant and resided in Canada at the time of recruitment; they were also 18 years of age or older, and able to read French, English, Spanish, Mandarin or Portuguese.
Recruitment
The CONCEPTION study uses diverse recruitment methodologies based on the WHO’s recent efforts to reach younger people where they get their information, namely social media.23 Recruitment is done on social media and the Internet, but also through obstetrics and gynecology departments in the Centre hospitalier universitaire (CHU) Sainte-Justine. Furthermore, recruitment is done in person in a community association of recent immigrants through the Montreal Diet Dispensary in Montréal, Quebec, which allows for the recruitment of individuals of lower socioeconomic status. Computer terminals are also made available to pregnant people at in-person recruitment sites. Finally, quick response codes are displayed on posters where in-person recruitment is done, in order for individuals to directly access the questionnaire with their mobile device, without having to be members of any of the social media platforms. With regards to our Web-based recruitment of pregnant people, a combination of social media platforms (Facebook, Instagram, Twitter, LinkedIn and TikTok) is used. Recruitment strategies and data intake questionnaires are available in French, English, Mandarin, Spanish and Portuguese, and postings are refreshed regularly (i.e., reposted weekly at the time of recruitment).
Survey design and development
Development of the questionnaire was done in English by J.G., Y.-H.G. and A.B., as well as the international team of collaborators working on the CONCEPTION study, as described previously.21,22 The questionnaire was translated into French and back-translated into English to ensure validity. The study instrument includes a number of validated questionnaires in all available languages. The questionnaire was pretested with 10 French-speaking and 10 English-speaking pregnant people and took an average of 25 minutes to complete. Participants were not compensated for their time.
To reach a diverse group of pregnant people, 2 team members (J.G. and Y.-H.G.) actively work on promoting the cohort on social media platforms via information segments (all platforms), mother–child and pregnancy support groups, outpatient and community clinics, and established hashtag strategies (Instagram, Twitter, LinkedIn), as well as through communication specialists affiliated with our team’s respective universities (all platforms including mainstream media — television, radio, press releases and news interviews). Recruitment via social media combined with in-person recruitment in times of crises is an appropriate methodology given the rapidity with which we need answers to pressing questions, such as how pregnant women are doing during the COVID-19 pandemic.24 Social media recruitment with anonymized data (survey, cross-sectional samples) has been used in other similar studies.8,25,26
Study variables
Maternal characteristics and general medical and peripartum medical history
Variables collected for the CONCEPTION study included age, gestational age, prepregnancy weight and ethnicity; sociodemographic characteristics (education, household annual income and marital status), place of residence (urban, suburban, rural), current employment status (type of work, working from home, still working on site and at what frequency); lifestyle behaviours during pregnancy, including smoking, alcohol, illicit drugs, cannabis products and multivitamin use; health status and medication use, such as prescribed medication use, comorbidity history (asthma, hypertension, diabetes, hyperthyroidism, hypothyroidism, dyslipidemia, depression, anxiety); pregnancy history, including parity; and COVID-19 diagnoses or symptoms (at the time of survey completion).
Vaccine variables
Participants were asked, “If it were available to you, would you accept the COVID-19 vaccine during your pregnancy?” If they said yes, we asked them whether they had been vaccinated against SARS-CoV-2 since the beginning of their pregnancy. Respondents had space to indicate they had booked an appointment and as such were categorized as “intended to be vaccinated.” Participants were also asked whether they had received the influenza vaccine during the 2020/21 season, which was analyzed as a proxy for acceptance of prepandemic public health measures. Additionally, participants were asked to assess their knowledge of COVID-19 severity in pregnancy, and of the safety and efficacy of the SARS-CoV-2 vaccine, in general and in pregnancy specifically, on a Likert scale ranging from 1 (no knowledge) to 5 (excellent knowledge). Of note, this is self-reported knowledge, which incorporates personal biases as well as past and present experiences.27,28 Each participant was further asked about their personal history of COVID-19. Finally, in cases of nonvaccination, the participant stated the reason(s) for their decision. Participants were provided with a list of reasons to select to explain why they were not vaccinated. They also had the option to enter other reasons in free-form text, which were compiled by our team and categorized.
Data analysis
Comparisons were performed according to vaccination status (vaccinated or intention to be vaccinated, which were pooled into 1 group; not vaccinated). Given the use of vaccination during pregnancy in other countries, and in anticipation of the SOGC statement supporting the use of all available and approved SARS-CoV-2 vaccines in Canada during any trimester of pregnancy on Apr. 20, 2021, our survey began capturing vaccination data on this date. The earlier recruits planned to get vaccinated but had not yet been vaccinated at the time of enrolment. As such, those who had the intention to be vaccinated and those who had been vaccinated were pooled together in our primary analyses. For all variables, comparisons using means with standard deviations (SDs) or proportions with ranges were performed, depending on whether the variables were continuous or categorical, using a Student t test or χ2 statistic, respectively. Self-reported knowledge scores were compared between groups using a Wilcoxon rank-sum test.
We quantified the determinants of SARS-CoV-2 vaccination during pregnancy with crude and adjusted logistic regression models, considering maternal age, annual household income and years of education as adjustment variables for each determinant. We calculated odds ratios (ORs) and 95% confidence intervals (CIs). We also calculated absolute and marginal risk difference, which were based on the adjusted logistic regressions.29 All statistical analyses were performed using R statistical software (version 4.1.0).
Ethics approval
This study was approved by the CHU Sainte-Justine’s research ethics committee (institutional ethical review approval no. 2021-2973).
Results
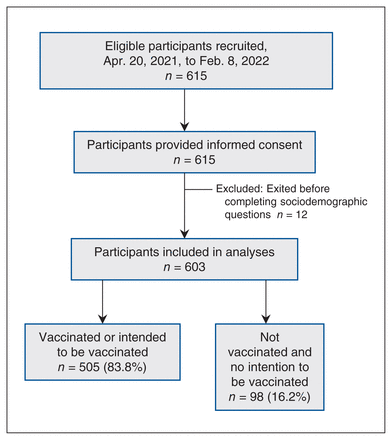
We recruited 615 participants into the CONCEPTION study from Apr. 20, 2021, to Feb. 8, 2022. Among these participants, 12 exited the survey without answering the sociodemographic questions. In the CONCEPTION study overall, participants took an average of 24 minutes, 39 seconds to complete the survey. A total of 603 participants were included in the analysis, of which 83.7% (n = 505) were vaccinated or had the intention to be vaccinated, and 16.3% (n = 98) were not vaccinated against SARS-CoV-2 and did not intend to be (Figure 1).
CONCEPTION study flowchart.
Cohort characteristics
The mean age of participants was 33.5 (SD 5.5) years at recruitment (Table 1). The mean gestational age at inclusion was 22.5 (SD 9.3) gestational weeks. Analyses showed significant differences in gestational age at inclusion according to vaccination status. Individuals who were vaccinated or had the intention to be vaccinated were recruited earlier in their pregnancy than nonvaccinated participants (22.0 [SD 9.4] v. 25.7 [SD 8.8] weeks’ gestation, p < 0.001) (Table 1). The prevalence of influenza vaccination for the 2020/21 season was significantly higher among individuals vaccinated against SARS-CoV-2 and those intending to be vaccinated (40.2%) than among individuals who were not vaccinated (10.2%, p < 0.001). Furthermore, vaccinated individuals and those intending to be vaccinated had more years of education than participants who were not vaccinated (17.4 v. 15.0 yr, respectively; p < 0.001). Pregnant people who were vaccinated or intended to be vaccinated had higher annual family income than nonvaccinated participants (p < 0.001) (Table 1). Sociodemographic data for all groups separately (i.e., intention to be vaccinated, vaccinated and nonvaccinated) are presented in Appendix 2 (available at www.cmajopen.ca/content/10/4/E1034/suppl/DC1).
Characteristics of study participants (recruited Apr. 20, 2021–Feb 8, 2022), stratified by SARS-CoV-2 vaccination status
Self-assessed knowledge of COVID-19 and predictors of vaccination status
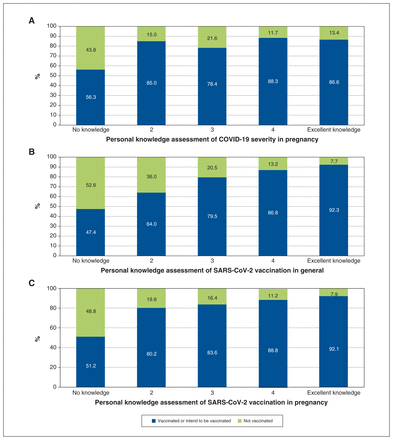
Vaccinated participants were more likely to report a higher prevalence of good (4/5) or excellent knowledge (5/5) on COVID-19 severity in pregnancy than nonvaccinated participants (p < 0.01, Figure 2A). This was also observed in the self-assessed knowledge of SARS-CoV-2 vaccination in general (p < 0.01, Figure 2B) and the self-assessed level of knowledge of SARS-CoV-2 vaccination in pregnancy (p < 0.01, Figure 2C). Participants who were not vaccinated reported lower self-assessed knowledge scores overall (Figure 2).
Personal knowledge assessment of (A) COVID-19 severity in pregnancy, (B) SARS-CoV-2 vaccination in general and (C) SARS-CoV-2 vaccination in pregnancy, stratified by vaccination status. Wilcoxon rank-sum test for differences between groups, p < 0.01.
Personal experience with COVID-19 stratified by vaccination status is shown in Table 2. Participants who were vaccinated or intended to be vaccinated reported more frequent testing for SARS-CoV-2 (62.0%) than nonvaccinated participants (55.1%), but this difference was not significant (p = 0.2) (Table 2). Receipt of an influenza vaccine within the most recent flu season was a significant predictor of being vaccinated or intention to be vaccinated (marginal risk difference 3.2%, 95% CI 3.0% to 3.3%, and adjusted OR 4.43, 95% CI 2.32 to 9.25) (Table 3). Being employed was significantly associated with being vaccinated or the intention to be vaccinated after adjustment for the other predictors (marginal risk difference 11.2%, 95% CI 10.6% to 11.9%, and adjusted OR 2.17, 95% CI 1.03 to 4.35) when compared with unemployment or receiving welfare support (Table 3). Self-assessed knowledge of COVID-19 severity in pregnancy and of SARS-CoV-2 vaccines in pregnancy and in general were not significant predictors of vaccination status, regardless of the level of self-reported personal knowledge (Table 3).
Personal experience with COVID-19
Predictors of vaccination status or intention to be vaccinated
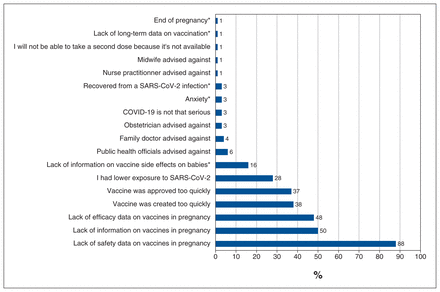
The main reasons stated for not being vaccinated against SARS-CoV-2 were lack of safety and efficacy data in pregnancy, the speed of vaccine creation and approval, and lack of information on vaccines in pregnancy (Figure 3). Reasons for nonvaccination submitted in free-form text included lack of information on vaccine adverse effects on babies and anxiety. Additionally, respondents also stated that public health officials, or their obstetrician, family doctor or midwife had advised against vaccination (Figure 3).
Reasons provided for nonvaccination (n = 98). *Reasons reported by participants in addition to those suggested in the survey.
Interpretation
In this study on SARS-CoV-2 vaccine hesitation and acceptance of SARS-CoV-2 vaccination in a sample of pregnant people in Canada, most individuals (83.7%) were vaccinated or intended to be vaccinated. After adjustment for maternal age, years of education, body mass index and annual household income, we identified that previous influenza vaccination uptake and employment status were significant predictors of SARS-CoV-2 vaccination among participants. The main reasons provided among individuals who were not vaccinated were lack of safety and efficacy data in pregnancy, and hasty approval of the vaccine.
Overall, we identified predictors for vaccination status, such as higher socioeconomic status (e.g., household income, years of education) and having been vaccinated during the last flu season, which is consistent with the literature.30
Influenza vaccination acceptance was considered a predictor of SARS-CoV-2 vaccination among the participants. Indeed, the group not vaccinated against SARS-CoV-2 reported lower influenza vaccination during the previous flu season. This result is consistent with vaccine hesitancy, a growing problem in public health over the last decade.31 In Canada, a vaccination survey tracks coverage every 2 years for all vaccines recommended by the National Advisory Committee on Immunization.32 Among pregnant people in 2019, only 45% were vaccinated against the flu and 44% against pertussis, and 3%–10% did not know if they had been vaccinated.19 With a prevalence of SARS-CoV-2 vaccination of 78.6% (474/603), in addition to 5.1% (31/603) who accepted vaccination but had not yet received a vaccine when they completed the survey, and a prevalence of flu vaccination of 35.7% overall, our reported vaccination acceptance is consistent with Canadian and international observations. 33,34 This discrepancy with our results may stem from the fact that vaccination rollout occurred at different times in Canada, with earlier access granted to pregnant people in Quebec than in Ontario.
Underestimation of vaccine efficacy and lack of trust were previously reported as 2 main reasons for vaccine hesitancy. 35,36 In our study, mistrust of the vaccine safety and efficacy were the most frequent reasons for nonvaccination. The population expressing vaccine hesitancy denounced a precipitous approval of vaccines, which suggests a lack of understanding of the approval process, lack of confidence in the procedures for validating vaccination in pregnant people, and a mistrust of national recommendations. The exclusion of pregnant people from vaccine safety trials37 did not prevent the SOGC from recommending the use of the SARS-CoV-2 vaccine for pregnant people.8 A recent surveillance review of the safety of mRNA SARS-CoV-2 vaccines during pregnancy indicated no significant outcomes among pregnant people who received the vaccine.38 Nonvaccination associated with a good to excellent self-assessment of knowledge level highlights the priority to address remaining knowledge gaps and confront the existing misinformation regarding SARS-CoV-2 vaccines. Indeed, while the vaccine validation trials were completed and many studies have provided reassuring SARS-CoV-2 vaccine safety data,1,38,39 patients mentioned inconsistent arguments to justify their vaccine hesitation, which may denote the limited access to evidence-based medicine in a constantly evolving and learning science. Additionally, finding 20% of individuals choosing nonvaccination because they perceived their risk of infection to be low highlights the importance of further research to explore context-specific barriers to vaccination in pregnancy.40
This study emphasizes the impact of educational strategies on behavioural determinants of health. Our findings suggest that the involvement of the patient in the preventive strategy (namely, vaccination) and the knowledge of the benefits and risks of the therapeutic plan generate better compliance and adherence to national public health recommendations. Since women’s health authorities have recommended that pregnant people discuss vaccination decisions with their health care providers,41,42 these findings emphasize the need for public health messaging and access to educational material (e.g., decisional flowcharts, brief reading material) to improve SARS-CoV-2 vaccination acceptance adapted to each distinct population. Health care providers are the most frequently trusted source of vaccine information and play a key role in shaping maternal attitudes toward vaccination.43 Although education on the safety of SARS-CoV-2 vaccination in pregnancy is essential, our results show that an alternative approach may be necessary to increase acceptance of SARS-CoV-2 vaccination, especially for those who experience mistrust in the health care system.
This study has several strengths, including a well-established recruitment strategy tailored to our study population and a large sample over an important period during the COVID-19 pandemic.
We measured self-assessed knowledge in lieu of being able to question individuals on their understanding of the vaccines and COVID-19. This parameter is used in the context of understanding personal medical choices, such as vaccination, as it accounts for personal biases and experiences.27,28 Given that the survey was anonymized, we do not believe this introduced a social-desirability bias.
Data intake was performed electronically, which allowed us to collect data in real time. We ensured the quality of data through complete data cataloguing.
Limitations
Limitations of the study include enrolment, in part, through social media, which may limit generalizability to those less familiar with social media and exclude those with lower socioeconomic status. However, the 2018 Canadian Internet Use Survey showed that more than 80% of Canadian adults aged 18–44 years use social media regularly (and > 90% adults aged 18–34 yr), women predominantly.44 Additionally, we acknowledge that our participants had higher-than-average household income compared with the Canadian population of the same age. Indeed, in 2019, the median household income for families with children was of $98 690, whereas the median salary bracket of our sample was $120 000–$150 000.45 As higher household income is thought to be a predictor of vaccine acceptance in the general population, our study population may be more inclined to accept vaccination.30 However, our recruiting team attempted to provide access to the study to pregnant people across social media groups, as well as through the Montreal Diet Dispensary. Additionally, the high SARS-CoV-2 vaccine uptake in our sample is representative of the vaccination uptake in Canada in the child-bearing age group, which is 88.60% in the 30–39 years age group (at least 1 dose) and 90% in the 18–39 years age group in Quebec.46 Lastly, the study design may have biased the selection of participants, preferentially recruiting individuals who were interested in the topic or favourable to vaccination, and who were more concerned about COVID-19; however, our methods did not target influencers, social media groups or news sources that were necessarily more provaccination than others. Of note, most (96.2%) of our study sample was from Quebec. Our study described the vaccine acceptance rate around the time that the mRNA vaccines were promoted.
Conclusion
Among the Canadian pregnant people who responded to this study, vaccine uptake against SARS-CoV-2 was high. However, our results underscore the importance of improving knowledge transfer about the efficacy of SARS-CoV-2 vaccines in pregnancy to guide vaccination efforts. Although prolonged monitoring is needed to evaluate late-onset neonatal and childhood outcomes associated with maternal SARS-CoV-2 vaccination, vaccination against SARS-CoV-2 in pregnancy is vital to controlling disease burden and decreasing morbidity in pregnancy. Given the economic, political, social and industrial repercussions, it is essential to consider the epidemiological factors influencing vaccine acceptance and prioritize their impact in public health and knowledge translation strategies. This study highlights the importance of adapted educational approaches to guide public health and research efforts as a key to improve vaccine acceptance in pregnant people. Indeed, the collaborative CONCEPTION study team is working on the development of educational tools and education strategies to reach pregnant people.
Acknowledgement
The authors thank the participants of the CONCEPTION study.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Anthony Atallah and Jessica Gorgui wrote the first draft of the manuscript. Jessica Gorgui and Yessica-Haydee Gomez actively recruited via social media and traditional media. Anick Bérard obtained funding, and obtained data and ethics approval. Jessica Gorgui performed the statistical analyses. All authors wrote the study protocol, interpreted the results and critically reviewed the manuscript. Jessica Gorgui and Anthony Atallah are co–first authors. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was funded by the Faculty of Pharmacy of the Université de Montréal.
Members of the CONCEPTION Study Group: Anaïs Lacasse, Sylvana Côté, Flory Muanda, Yves Mufike, Anne Monique Nuyt, Caroline Quach, Ema Ferreira, Padma Kaul, Brandace Winquist, Kieran J. O’Donnell, Sherif Eltonsy, Dan Chateau, Gillian Hanley, Tim Oberlander, Behrouz Kassai, Sabine Mainbourg, Sasha Bernatsky, Évelyne Vinet, Annie Brodeur-Doucet, Jackie Demers, Philippe Richebé and Valerie Zaphiratos
Data sharing: Institutional contacts for data access: CHU Sainte-Justine Ethics Committee (http://chusj.nagano.ca), Montréal, Quebec, for researchers who meet the criteria for access to confidential data.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/10/4/E1034/suppl/DC1.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/
References
- © 2022 CMA Impact Inc. or its licensors