Abstract
Background: Urinary incontinence affects up to half of women, yet few speak to their health care provider about or receive treatment for the condition. To aid with identifying subpopulations at risk for urinary incontinence, we examined the associations between 10 chronic health conditions and urinary incontinence among Canadian adult females.
Methods: We conducted a cross-sectional analysis of survey data from the Canadian Community Health Survey (2013–2014) involving female respondents aged 25 years or older living in a private dwelling. Presence of chronic conditions and urinary incontinence were measured by self-report. We used logistic regression modelling with sampling weights, controlling for age, income, ethnicity, body mass index and smoking. Multiple imputation and probabilistic bias analysis were used to address missing covariate data and unmeasured confounding from parity.
Results: Our analysis included 60 186 respondents representing more than 12 million Canadian females, of whom 45.8% (95% confidence interval [CI] 45.0%–46.6%) reported at least 1 chronic condition. Chronic conditions were associated with more than twice the odds of urinary incontinence (adjusted odds ratio [OR] 2.42, 95% CI 2.02–2.89). Associations were largest for bowel disorders (adjusted OR 2.92, 95% CI 2.44–3.49); modest for chronic obstructive pulmonary disease (adjusted OR 2.00, 95% CI 1.63–2.45), asthma (adjusted OR 1.82, 95% CI 1.52–2.19), arthritis (adjusted OR 1.98, 95% CI 1.74–2.24) and heart disease (adjusted OR 1.73, 95% CI 1.48–2.02); and smallest for diabetes (adjusted OR 1.20, 95% CI 1.02–1.41) and high blood pressure (adjusted OR 1.27, 95% CI 1.12–1.44). Results slightly attenuated but did not substantively change after imputation and bias analysis.
Interpretation: We found that chronic conditions are associated with significantly higher odds of comorbid urinary incontinence among Canadian adult females, which is consistent with previous research. Our findings support routine inquiry regarding urinary incontinence symptoms among women accessing health care for chronic conditions.
Urinary incontinence (the involuntary leakage of urine) affects up to half of women.1–3 Urinary incontinence can be divided into 3 predominant types: stress urinary incontinence with increased abdominal pressure (about 50% of cases), urgency urinary incontinence as the sudden need to urinate (about 10%), and mixed urinary incontinence with stress and urgency symptoms (about 30%).4 Detailed classification into 9 subtypes exists, with a multitude of storage, sensory and voiding symptoms that can overlap and coexist in the presentation of urinary incontinence.4 Most women experience persistent symptoms up to a decade after onset.5 Well-being and daily function are profoundly affected by urinary incontinence, including higher odds of anxiety and depression,6 lower quality of life,7 diminished self-esteem,8 and avoidance of social activities and intimacy.9–11
Treatments include behavioural approaches, pharmacotherapy and surgical correction, with the latter generally reserved for women who do not achieve adequate symptom relief with the former, conservative methods.12 However, more than 60% of women with urinary incontinence do not seek treatment because of embarrassment, perceptions that urinary incontinence is a normal consequence of aging or childbirth, or lack of awareness regarding treatment.13 Those seeking treatment report more severe symptoms, living with urinary incontinence for several years and lower quality of life.14,15 This suggests that symptoms may go untreated initially, worsen over time, or reach a threshold of bother as women age or accumulate comorbidities that prompts help-seeking. Provider-initiated discussions about pelvic floor function among women at risk for urinary incontinence can aid in proactively identifying and treating women experiencing leakage.16
Women with chronic physical health conditions appear to be at higher risk of urinary incontinence.1,3,17–19 Melville and colleagues reported that medical comorbidity was a significant risk factor for urinary incontinence at least monthly among women aged 20 years and older, with an odds ratio (OR) of 1.34 (95% confidence interval [CI] 1.13–1.59).1 Tennstedt and colleagues found 1.8 times the odds of weekly urinary incontinence among women with asthma (95% CI 1.12–3.00) and arthritis (95% CI 1.15–3.00), but no association with diabetes, heart disease or hypertension.18 Conversely, Daugirdas and colleagues found mixed urinary incontinence during the past 12 months to be associated with hypertension (OR 1.31, 95% CI 1.02–1.60), cardiovascular disease (OR 1.62, 95% CI 1.26–2.10) and diabetes (OR 1.61, 95% CI 1.28–2.01), after adjusting for age, ethnicity, body mass index (BMI) and parity. 19 Studies on irritable bowel syndrome (IBS) have mixed conclusions, with some reporting higher prevalence of urgency urinary incontinence in affected women.20,21 These trends are reflected in studies involving individuals living with urinary incontinence, who generally experience a disproportionately higher physical and emotional comorbid load and greater risk of functional limitations.17,22
Notwithstanding, there are some inconsistencies in the magnitude and statistical significance of associations between urinary incontinence and diabetes, hypertension and cardiovascular disease, and scant population-based studies exist on the relation between incontinence and rheumatic, gastrointestinal and respiratory conditions. Existing research is largely set in the United States and may not reflect the experience of Canadians with differing health care access and prescription drug coverage for chronic conditions. Therefore, we examined the associations between a range of 10 chronic physical health conditions and urinary incontinence among Canadian adult females.
Methods
Study design and data source
We conducted a cross-sectional analysis using public use microdata files from the 2013 and 2014 cycles of the Canadian Community Health Survey (CCHS). The CCHS is a population-based, national survey that collects self-reported information on health outcomes and determinants in the Canadian household population, excluding those living in institutions, those living on Indigenous reserves and full-time members of the Canadian Armed Forces (< 3% of the population). Details on the CCHS multistaged, stratified sampling design and computer-assisted survey data-collection methodology are available from Statistics Canada.23,24 In total, 147 009 out of 193 813 households (75.9% response rate) and 128 310 individuals from each of those responding households (87.3% response rate) participated in the 2013–2014 CCHS.24
Participants
We included female respondents aged 25 years or older (the age at which the CCHS began asking about urinary incontinence) with complete data for urinary incontinence and chronic conditions.
Exposure and outcome variables
Our study exposure was chronic conditions and the outcome was urinary incontinence. Respondents were asked a series of binary (yes v. no) questions about the presence of chronic conditions and urinary incontinence after a preamble specifying that these must have been diagnosed by a health professional or expected to last (or have already lasted) 6 months or longer. Chronic conditions measured in the CCHS were asthma, arthritis, high blood pressure, diabetes, heart disease, bowel disorders, chronic obstructive pulmonary disease (COPD) and cancer. Individuals with diabetes or bowel disorder were asked a follow-up question about the type of disease they had: type 1 or type 2 for diabetes; inflammatory bowel disease (IBD; Crohn disease or ulcerative colitis) or IBS for bowel disorder.
Covariates
Potential confounders were identified on the basis of published literature,1,3,25,26 authors’ clinical expertise and availability in the CCHS public use data set. Body mass index category was derived by Statistics Canada using self-reported height and weight and classified by Statistics Canada as underweight (< 18.5), normal weight (18.5–24.9), overweight (25.0–29.9) or obese (≥ 30). Age was available in 5-year categories, from 25–29 years up to 80 years and older. Smoking status was derived by Statistics Canada using information on current frequency of cigarette use and classified as daily, occasionally (less than daily) or none. Ethnicity was available as white or visible minority based on Statistics Canada classification of self-reported cultural and racial origin. Household income was available by quintile at the provincial level. These social determinants of health have been associated with differences in the prevalence of both chronic disease and urinary incontinence.2,25
Obstetric history is strongly related to urinary incontinence, including parity, mode of delivery in previous births, and obstetric interventions such as episiotomy;1,25 at minimum, most studies on determinants of urinary incontinence adjust for parity. However, data on respondents’ obstetric history are not routinely collected and were therefore not available in the CCHS.
Statistical analysis
We described characteristics of females with and without chronic conditions using frequencies, proportions and standardized differences.27 To quantify the association between chronic conditions and urinary incontinence, we used 2 approaches to construct models for the presence of 1 or more chronic condition, for the presence of 2 or more chronic conditions (i.e., multimorbidity) and for each individual condition.
First, we used the conventional approach of logistic regression yielding ORs and 95% CIs and adjusting for the a priori confounders of ethnicity, smoking status, income quintile, BMI category and age. Income and ethnicity were assessed as potential effect modifiers using interaction terms; in the absence of statistical evidence for modification, they were included as covariates.
Second, we used a combination of multiple imputation with chained equations (MICE) and probabilistic bias analysis to sequentially address the missing covariate data and unmeasured confounding. MICE was used to impute missing values for all covariates except age (for which complete data were available), with 15 imputations performed using Stata’s mi command suite (Appendix 1A, available at www.cmajopen.ca/content/10/2/E296/suppl/DC1). Auxiliary variables were included in covariate imputation models if correlations were 0.1 or greater.28
Probabilistic bias analysis was used to address unmeasured confounding from parity (nulliparous, parous) to improve the comparability and validity of our estimates relative to existing literature on female urinary incontinence (Appendix 1B).29–31 Parity is a moderate risk factor for urinary incontinence, with the odds of urinary incontinence almost doubled in women who have given birth compared with those who have not.32 Evidence on the association between parity and chronic conditions is limited, but it does suggest that the prevalence of having children is up to 22% lower in affected women.33–36 Thus, omitting parity from the analysis may result in confounding of the OR between chronic conditions and urinary incontinence. Previous work has shown that the size of this confounding bias, termed the relative risk due to confounding (RRC), can be estimated using 3 values: the prevalence of the confounder in both the exposed and unexposed, and the association between the confounder and the outcome.30 We estimated these 3 values using published data and clinical expertise. Then, following the methods by Lash and colleagues, we parameterized a normal distribution for the RRC and used Monte Carlo methods to adjust the ORs (from MICE models) for parity using a simulation of 10 000 iterations.29 This process enabled us to quantify the ORs that would have been observed had data on respondent parity been available, and rests on the correctness of the assumed input values for RRC. Random error from the conventional analysis was combined with systematic error from the simulation to compute the total error interval.
For all analyses, we applied sampling weights derived by Statistics Canada, which account for the complex survey design (i.e., probability of selection, nonresponse) and enable accurate weighted point estimates but conservative variance estimates.24 Data were analyzed in Stata IC Version 15.
Ethics approval
In accordance with the Canadian Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans Article 2.2, this secondary analysis using publicly available CCHS data was exempted from ethical review and approval.
Results
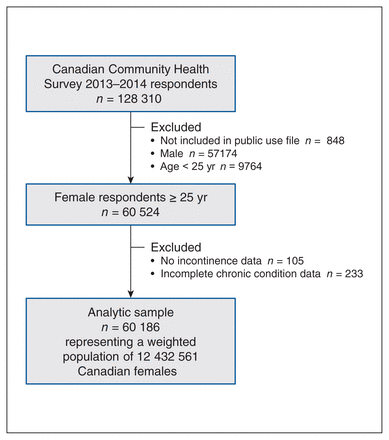
Our sample included 60 186 female respondents aged 25 years or older; when weighted, this represented 12 432 561 females in the Canadian household population (Figure 1). Table 1 displays sample characteristics stratified by chronic condition status. Most females with 1 or more chronic condition were aged 55 years or older, whereas females without a chronic condition were predominantly younger than 55. Females with a chronic condition were less likely to have household income in the higher quintiles and more likely to be overweight or obese; a slightly larger proportion self-identified as white.
Selection of Canadian Community Health Survey 2013–2014 respondents for this analysis.
Characteristics of Canadian females aged 25 years and older stratified by self-report of 1 or more chronic conditions*
Prevalence of chronic conditions and urinary incontinence
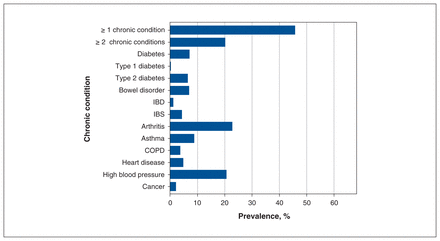
Figure 2 shows the weighted prevalence estimates for chronic conditions (numeric results are available in Appendix 1, Table C1). Overall, 45.8% (95% CI 45.0%–46.6%) of females reported 1 or more chronic condition, and 20.2% (95% CI 19.6%–20.7%) reported 2 or more conditions. Conditions with the highest prevalence were arthritis (22.8%) and high blood pressure (20.7%). Table 2 includes the prevalence of urinary incontinence among females with and without each chronic condition. Urinary incontinence was prevalent in 10.0% of females with a chronic condition, and only 2.1% of females without a condition. Urinary incontinence was most prevalent among females with COPD, heart disease and bowel disorder.
Prevalence of chronic conditions in Canadian females aged 25 years and older. Diabetes includes both type 1 or type 2, and bowel disorder includes both IBD and IBS; some individuals with these conditions did not specify their type of disease. Estimates are weighted to represent the Canadian household population. Note: COPD = chronic obstructive pulmonary disease, IBD = inflammatory bowel disease (Crohn disease or ulcerative colitis), IBS = irritable bowel syndrome.
Association between chronic conditions and urinary incontinence in Canadian females aged 25 years and older*
Logistic regression analysis
Table 2 shows associations between chronic conditions and urinary incontinence, using a conventional logistic regression approach and a combined MICE and bias analysis approach (the full results from each modelling stage are available in Appendix 1, Table C2). There was no evidence of effect modification by income and ethnicity. In the conventional approach, presence of 1 or more chronic condition was associated with more than twice the odds of urinary incontinence (adjusted OR 2.42, 95% CI 2.02–2.89), as was presence of 2 or more conditions (adjusted OR 2.10, 95% CI 1.82–2.42).
Most conditions were associated with greater odds of urinary incontinence, except for type 1 diabetes (adjusted OR 1.03, 95% CI 0.40–2.62) and cancer (adjusted OR 0.90, 95% CI 0.69–1.16). Among individual conditions, bowel disorders were most strongly associated with urinary incontinence, with an adjusted OR of 2.92 (95% CI 2.44–3.49); the strength of this association persisted across subtypes IBD (adjusted OR 3.11, 95% CI 1.82–5.33) and IBS (adjusted OR 2.80, 95% CI 2.27–3.46). Modest associations were observed for COPD (adjusted OR 2.00, 95% CI 1.63–2.45), arthritis (adjusted OR 1.98, 95% CI 1.74–2.24), asthma (adjusted OR 1.82, 95% CI 1.52–2.19) and heart disease (adjusted OR 1.73, 95% CI 1.48–2.02). Smaller associations were observed for type 2 diabetes (adjusted OR 1.17, 95% CI 0.99–1.38) and high blood pressure (adjusted OR 1.27, 95% CI 1.12–1.44).
Multiple imputation and bias analysis
Results from the conventional approach were comparable to those obtained using MICE and bias analysis. Adjusted ORs were attenuated for most conditions, with the degree of attenuation ranging from 2.5% to 18.0% (mean attenuation 9.4%), and the width of 95% CIs was largely consistent.
Interpretation
Using nationally representative survey data, we found that Canadian females aged 25 years and older with chronic conditions had more than twice the odds of comorbid urinary incontinence. Associations were largest for bowel disorders, modest for respiratory and cardiovascular diseases and arthritis, and smallest for diabetes and hypertension. Use of quantitative methods to address missing data and unmeasured confounding slightly attenuated results (by an average of 9%), but interpretations were unchanged.
Our findings reaffirm that chronic conditions are a significant risk factor for urinary incontinence in women. Previous studies using a composite measure of chronic conditions have reported ORs ranging from 1.2 to 1.5,1,26 which are smaller than the adjusted OR of 2.42 we report here. Variability in definitions of urinary incontinence (sometimes as broad as leaking even a small amount in the past 12 months)26 and the list of conditions may explain this discrepancy.
We found that females with bowel disorders have more than 2.5 times the odds of urinary incontinence. Irritable bowel syndrome is frequently comorbid with urogenital symptoms, and central sensitization (i.e., increased responsiveness to stimuli in the central nervous system) present in IBS is thought to invoke bladder overactivity or smooth muscle dysfunction.37,38 Comparatively, there is a dearth of published data on urinary symptoms in IBD. Our findings are consistent with knowledge of pelvic floor physiology in bowel disorders. Cross-sensitization (“cross-talk”) between neural pathways in pelvic organs provides a pathway for shared dysfunction, and common pelvic floor musculature supporting the bladder and bowel may be damaged by chronic straining, incontinence or diarrhea.39 Indeed, bowel symptoms such as constipation and fecal incontinence occur frequently in women with urinary incontinence.40,41
Our adjusted ORs for asthma, COPD, heart disease and arthritis align with findings from previous studies, corroborating a 1.5- to 2.5-fold association with urinary incontinence. 18,42 Chronic coughing likely explains increased urinary incontinence among women with asthma and COPD; the rapid increase in intra-abdominal pressure and impact loading on pelvic musculature and connective tissue can create damage over time.43 Heart disease includes a variety of cardiovascular conditions, and thus physiologic links with urinary incontinence may vary. In heart failure, for example, urinary incontinence may result from increased urgency and frequency of nighttime urination related to compensatory increases in circulating natriuretic peptide or redistribution of interstitial edema when sleeping in a supine position.44 Mobility issues such as reaching the bathroom on time are thought to explain urinary incontinence in women with arthritis,45 though we additionally suspect the role of central sensitization, which has been observed in osteoarthritis and arthralgias.37
Across all conditions, the presence of urinary incontinence as an adverse effect of medications is unclear. Battaglia and colleagues reviewed trials on inhaled therapy in COPD and found that none explored urinary incontinence as an adverse effect.46 Heart disease medications such as β-blockers and loop diuretics are thought to increase bladder contractility through inhibiting sympathetic nervous system activation and increasing the volume of urine, respectively, though data supporting this effect are inconclusive.47,48
Studies on diabetes and urinary incontinence have ranged from detecting a small25,49 or nonsignificant18,50 association (ORs from 1.1 to 1.3) to large associations in excess of twofold. 42,51 Our sample composition and model covariates were most similar to those of Ebbesen and colleagues49 and Løwenstein and colleagues,50 and results are remarkably consistent — an OR of 1.20 in our study, 1.21 by Ebbesen and 1.11 by Løwenstein. Our analysis suggested that this association may be driven by type 2 more than type 1 diabetes. The classic mechanism of urinary incontinence in diabetes involves loss of autonomic innervation of the bladder (neuropathy), which has implications for both subtypes.52 However, women with type 2 diabetes likely have additional risk factors for urinary incontinence, including elevated BMI and metabolic abnormalities.53,54 Newer oral medications in type 2 diabetes induce osmotic diuresis by increasing glucose excretion in the kidney,55 and the resulting increase in urinary volume and frequency may in theory promote urinary incontinence. Although the OR for type 2 diabetes in the current study was not statistically significant, the CI values indicate that either a null or small association with higher odds of urinary incontinence are compatible with our data. Additional studies are warranted to replicate and confirm this finding.
Our findings, together with several plausible mechanisms underpinning the associations we observed, indicate that health care providers should routinely inquire about urinary incontinence when women access health care for chronic conditions. In one study, only 3% of women with urinary incontinence reported that their provider initiated a discussion about leakage, and 55% reported discussing leakage with their provider at all.56 Qualitative work has consistently found that women would prefer that their health care providers initiate discussions about urinary incontinence, because embarrassment, worry and stigma serve as barriers to self-initiated disclosure.57
Urinary incontinence can impair women’s quality of life and daily functioning on its own, but when comorbid with chronic conditions, it may pose unique challenges for self-management. For example, women may be less prone to engage in lifestyle behaviours like physical activity that can aid in managing symptoms of chronic conditions but exacerbate urinary incontinence. Medications perceived to promote urinary incontinence may be taken inconsistently or stopped altogether. Discussions about urinary incontinence are therefore important for overall quality of life, but also to ensure disease management plans are appropriately tailored and optimized in tandem with urinary incontinence symptoms.58
Early detection of urinary incontinence may promote earlier resolution of leakage, reduce the need for invasive intervention and prevent the psychosocial burden of symptoms. 59 In light of known suboptimal assessment of urinary incontinence by health care providers, research and education on assessment barriers and implementation strategies are warranted.
Limitations
The CCHS is cross-sectional; thus, we cannot establish temporality between diagnosis of chronic conditions and onset of urinary incontinence. Self-reported measurement of chronic conditions is imperfect relative to clinical examinations or medical record information such that a small-to-moderate degree of misclassification bias in our analysis is likely. A previous validation study of self-reported chronic conditions in the CCHS compared with administrative data found that misclassification was generally due to underreporting,60 suggesting that our estimates may be conservative.
Type and severity of urinary incontinence were not measured, and it is possible that certain chronic conditions are differentially associated with specific urinary incontinence pathologies and not others; however, the preamble used in the CCHS survey ensures that reported urinary incontinence has a degree of bother or chronicity, and thus, the associations we reported here represent the average effects of chronic conditions on urinary incontinence from a public health perspective. The CCHS public use data set lacks data on obstetric and surgical history and detailed information on race or ethnicity, which may be important sources of unmeasured confounding.
Conclusion
We found that chronic conditions were associated with more than twice the odds of urinary incontinence among Canadian females. Associations were large for bowel disorders; modest for respiratory conditions, cardiovascular disease and arthritis; and small for diabetes. Findings are consistent with several disease pathology and treatment mechanisms through which chronic conditions and urinary incontinence may co-occur. Early identification and intervention for urinary incontinence initiated by health care providers may be a necessary addition to clinical care for women with chronic conditions.
Footnotes
Competing interests: Cynthia Seow has served on advisory boards for Janssen, AbbVie, Takeda, Ferring, Shire, Pfizer, Sandoz, Pharmascience, Fresenius Kabi and Amgen, and as a speaker for Janssen, AbbVie, Takeda, Ferring, Shire, Pfizer and Pharmascience. Erin Brennand has served as a speaker for SearchLight Pharmaceuticals. These connections are outside of the current work. No other competing interests were declared.
This article has been peer reviewed.
Contributors: All authors have materially participated in this research. Natalie Scime conceived the study concept and drafted the data analysis plan under the supervision of Erin Brennand, and with feedback from Erin Hetherington, Amy Metcalfe and Kathleen Chaput. Natalie Scime conducted the analysis with methodologic support from Erin Hetherington. Natalie Scime and Erin Brennand wrote the initial draft of the manuscript; Erin Hetherington, Amy Metcalfe, Kathleen Chaput, Sandra Dumanski and Cynthia Seow contributed to interpretation of the data and critically reviewed the manuscript. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: Natalie Scime is supported by a Canadian Institutes of Health Research (CIHR) Doctoral Award. Erin Hetherington is supported by a CIHR Post-doctoral Fellowship. Amy Metcalfe is supported by a CIHR New Investigator Award.
Data sharing: Data from the Canadian Community Health Survey are publicly accessible on request to and approval from Statistics Canada.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/10/2/E296/suppl/DC1.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/
References
- © 2022 CMA Impact Inc. or its licensors