Abstract
Purpose: To use data from the Randomized Aldactone Evaluation Study (RALES) to compare clinical outcomes and costs as part of the assessment of the economic implications of spironolactone treatment of advanced heart failure.
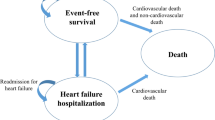
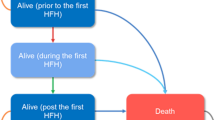
Methods: RALES was a randomized, double-blinded, placebo-controlled trial that enrolled participants who had severe heart failure and a left ventricular ejection fraction of no more than 35% and who were receiving standard therapy, including an angiotensin-converting enzyme inhibitor, a loop diuretic, and, in some cases, digoxin. We used a decision analytic model that incorporated data from participants in RALES as well as cost data from five countries that participated in the study. Costs were calculated for nonfatal hospitalizations, ambulatory care, spironolactone therapy, and death. The primary health outcome was quality-adjusted life-years saved (QALYS). Outcomes were evaluated for the first 35 months of observation in RALES.
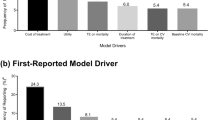
Results: Spironolactone therapy during the first 35 months of follow-up in RALES increased quality-adjusted survival time (0.13 QALYS, 95% CI, 0.07 to 0.18) without increasing costs ($713 savings, 95% CI, $2123 savings to $783 in costs). Spironolactone therapy either dominated placebo or had a ratio of cost per QALYS that was unlikely to exceed $20,300. These results were robust in both one-way and multiway sensitivity analyses.
Conclusions: Even after implementation of current clinical guidelines, addition of spironolactone therapy provides an opportunity to further reduce the large clinical and economic burden of patients with heart failure.
Similar content being viewed by others
References
Rich MW, Epidemiology, pathophysiology, and etiology of congestive heart failure in older adults. J Am Geriatr Soc 1997;45:968–974.
American Heart Association. 1999 Heart and Stoke Statistical Update. Dallas:Texas: American Heart Association, 1999.
Gillum RF. Epidemiology of heart failure in the United States. Am Heart J 1993;126:1042–1047.
Daley J, Jencks S, Draper D, Lenhart G, Thomas N, Walker J. Predicting hospital-associated mortality for Medicare patients. JAMA 1988;260:3617–3628.
Konstam MA, Dracup K, Baker DW, et al. Heart failure: Evaluation and care of patients with left-ventricular systolic dysfunction. Publication No. 94-06122. Rockville, MD:US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; June 1994.
O'Connell JB, Bristow MR. Economic impact of heart failure in the United States: Time for a different approach. J Heart Lung Transplant 1993;13:S107–S112.
Crowie MR, Mosterd A, Wood DA, et al. The epidemiology of heart failure. Eur Heart J 1997;18:208–225.
Consensus recommendations for the management of chronic heart failure. On behalf of the membership of the advisory council to improve outcomes nationwide in heart failure.Am J Cardiol 1999;83:1A–38A.
Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 1996;334:1349–1355.
Colucci WS, Packer M, Bristow MR, et al., for the US Carvedilol Heart Failure Study Group. Carvedilol inhibits clinical progression in patients with mild symptoms of heart failure. Circulation 1996;94:2800–2806.
CIBIS II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study (CIBIS-II): A randomised trial. Lancet 1999;353:9–13.
Hjalmarson A, Glodstein S, Fagerberg B, et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: The Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). JAMA 2000;283:1295-1302.
Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341:709–717.
Glick H, Cook J, Kinosian B, et al. Costs and effects of enalapril therapy in patients with symptomatic heart failure: An economic analysis of the Studies of Left Ventricular Dysfunction Treatment Trial. J Card Fail 1995;1:371–380.
Cook J, Glick H, Kinosian B, Heyse J. An assessment of “Ladder of Life” score as a value measure for health status in congestive heart failure (Abstract). Med Decis Making 1993;13:386.
Fleg JL, Hinton PC, Lakatta EG, et al. Physician utilization of laboratory procedures to monitor outpatients with congestive heart failure. Arch Intern Med 1989;149:393–396.
Lipscomb J, Weinstein MC, Torrance GW. Time preference. In: Gold MR, Siegel JE, Russell LB, Weinstein MC, eds. Cost-Effectiveness in Health and Medicine, Oxford: Oxford University Press, 1996:233.
Polsky D, Glick HA, Willke R, Schulman K. Confidence intervals for cost-effectiveness ratios: A comparison of four methods. Health Econ 1997;6:243–252.
Black WC. The CE plane: A graphic representation of cost effectiveness. Med Decis Making 1990;10:212–214.
Rubin DB. Inference and missing data. Biometrika1976;63:581–592.
Lin DY, Feuer EJ, Etzioni R, Wax Y. Estimating medical costs from incomplete follow-up data. Biometrics 1997;53:113–128.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Glick, H.A., Orzol, S.M., Tooley, J.F. et al. Economic Evaluation of the Randomized Aldactone Evaluation Study (RALES): Treatment of Patients with Severe Heart Failure. Cardiovasc Drugs Ther 16, 53–59 (2002). https://doi.org/10.1023/A:1015371616135
Issue Date:
DOI: https://doi.org/10.1023/A:1015371616135